
Alutard Sq

Ask a doctor about a prescription for Alutard Sq

How to use Alutard Sq
Package Leaflet: Information for the Patient
ALUTARD SQ
Suspension for injection
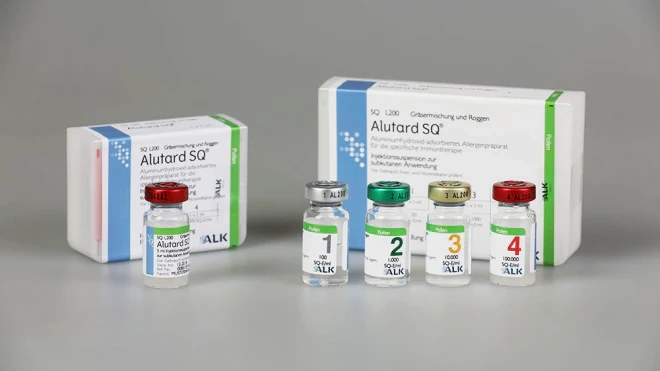
Basic treatment: 100 SQ-U/ml, 1000 SQ-U/ml, 10,000 SQ-U/ml, 100,000 SQ-U/ml
Maintenance treatment: 100,000 SQ-U/ml
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- Ask your doctor, pharmacist, or nurse if you have any questions.
- This medicine has been prescribed specifically for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any possible side effects not listed in this leaflet, tell your doctor, pharmacist, or nurse.
Table of Contents of the Leaflet:
- 1. What is ALUTARD SQ and what is it used for
- 2. Important information before using ALUTARD SQ
- 3. How to use ALUTARD SQ
- 4. Possible side effects
- 5. How to store ALUTARD SQ
- 6. Contents of the pack and other information
1. What is ALUTARD SQ and what is it used for
ALUTARD SQ is used to treat allergic diseases that are dependent on specific immunoglobulin E (IgE).
2. Important information before taking ALUTARD SQ
After the injection, the patient should remain in the medical facility for at least 30 minutes due to the possible occurrence of an allergic reaction.
When not to use ALUTARD SQ:
Consult your doctor, even if the above warnings apply to past situations.
Warnings and precautions
On the day of injection, avoid strenuous physical activity, hot baths, and alcohol consumption.
Before each allergen injection, the doctor should check the volume and date of the previous injection (the interval between doses).
Before starting treatment with ALUTARD SQ, tell your doctor if:
- A allergic reaction occurred after the last injection, as this may indicate the need to use a smaller dose (dose reduction).
- The patient is being treated for depression with tricyclic antidepressants, monoamine oxidase inhibitors (MAOIs), or is being treated for Parkinson's disease with catechol-O-methyltransferase (COMT) inhibitors.
- The patient has chronic heart or lung disease or kidney disease.
- The patient has a disease that affects the immune system or is taking medications that suppress immune system function.
- The patient is taking beta-blockers to reduce blood pressure.
- The patient has a fever or any other symptoms of infection.
- The patient has experienced symptoms of a severe allergic reaction, such as hay fever, asthma, or rash in the last few days before the injection.
After injecting ALUTARD SQ:
- After each injection, the patient should remain under the doctor's observation for 30 minutes. Symptoms of an allergic reaction may occur later than 30 minutes after the injection.
- If symptoms of a severe allergic reaction occur, such as hives, difficulty swallowing or breathing, voice changes, blood pressure drop, or a lump in the throat, seek medical help immediately.
- If asthma symptoms worsen, seek medical help immediately.
The dose of the injected medicine should be changed or the injection should be postponed if:
- A fever or other symptoms of infection occur
- Allergic symptoms occur within the last 3-4 days
- There is a significant decrease in lung function (based on the doctor's assessment)
- Previous adverse reactions (local or generalized) have occurred
- There is an exacerbation of atopic dermatitis
- A protective vaccine has been administered
ALUTARD SQ and other medicines
Tell your doctor about all medicines you are taking or have recently taken, as well as any medicines you plan to take.
Tell your doctor or healthcare professional about taking antiallergic medicines, such as antihistamines or corticosteroids, as they may affect possible side effects of this treatment. In this case, the doctor may adjust the dose.
In the case of other vaccinations, e.g., protective vaccinations, maintain an interval of at least one week before and after injecting ALUTARD SQ.
In the case of concurrent treatment with other allergens, injections should be administered sequentially in each arm. Wait at least 30 minutes between injections.
During treatment with ALUTARD SQ, avoid taking large amounts of aluminum-containing medications, such as some antacids.
Some medications may affect the action of adrenaline. Adrenaline is used to treat severe allergic reactions. Therefore, inform your doctor or healthcare professional about taking any of the following medications: beta-blockers to reduce blood pressure, tricyclic antidepressants, or monoamine oxidase inhibitors (MAOIs) used to treat depression or COMT inhibitors used to treat Parkinson's disease.
ALUTARD SQ with food and drink
Avoid consuming alcohol on the day of injection, as it may increase the risk of a severe allergic reaction (anaphylaxis) and worsen its severity.
Pregnancy and breastfeeding
Pregnancy
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before using this medicine.
Do not start treatment during pregnancy. Women who become pregnant during treatment with ALUTARD SQ may continue to use the medicine after the doctor has assessed their overall condition and reaction to previous doses.
Breastfeeding
It is not known whether ALUTARD SQ is excreted in breast milk. If you are breastfeeding, consult your doctor before starting treatment.
Driving and using machines
Treatment with ALUTARD SQ should not affect your ability to drive or use machines.
ALUTARD SQ contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per dose, which means the medicine is considered "sodium-free".
3. How to use ALUTARD SQ
This medicine should always be used as directed by your doctor. If you are unsure, consult your doctor or pharmacist.
Method of administration
Before administering the medicine, the ALUTARD SQ vial should be slowly turned upside down and back 10-20 times. The medicine is injected subcutaneously into the upper arm or forearm. It is recommended to alternate the injection site between the right and left arms during subsequent injections.
Dosage:
ALUTARD SQ may only be administered in medical facilities under the supervision of a doctor experienced in specific immunotherapy and in medical facilities where appropriate medications and equipment are available to treat potential anaphylactic reactions.
The patient must remain in the medical facility for at least 30 minutes after each injection.
Treatment is divided into two phases: the dose-increase phase (the dose is gradually increased) and the maintenance phase (a constant dose is used).
Dosage in both phases is individually determined by the attending physician, depending on the patient's tolerance and sensitivity to the allergen.
Dose-increase phase
During the initial phase, the allergen dose is increased to achieve the maximum tolerated dose, which is the maintenance dose.
During the initial phase, one injection is given per week for 13 weeks to achieve the maintenance dose.
Maintenance phase (maintenance treatment)
After achieving the maintenance dose, the interval between injections is gradually increased from 1 to 2, 4, and 6 weeks. Then, treatment is continued for 3 years, with injections every 6 weeks ± 2 weeks.
Dose reduction:
The doctor should adjust the dose of ALUTARD SQ in the following situations:
- if more time has passed since the last visit than recommended,
- in case of a severe reaction at the injection site, lasting more than 6 hours after the injection. Such a reaction should be reported to the attending physician,
- in case of a severe, generalized reaction to the medicine, the attending physician will consider whether to continue treatment,
- in case of continued treatment, the next dose will be reduced to 10% of the dose that caused the reaction.
Use in children and adolescents
ALUTARD SQ is not usually recommended for the treatment of allergies in children under 5 years of age.
Use of a higher than recommended dose of ALUTARD SQ
In case of accidental administration of too high a dose of ALUTARD SQ, there is an increased risk of allergic reactions. Therefore, remain in the medical facility for at least 30 minutes for observation. If necessary, treatment for potential side effects will be administered.
If you have any further questions about the use of this medicine, consult your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Ask your doctor what to do if you experience any side effects.
Seek medical attention immediately if you experience a severe side effect.
A side effect may be an allergic reaction to the allergen being treated.
Symptoms of side effects may occur within the first 30 minutes after the injection, although they may also appear within 24 hours after the injection.
Most side effects are mild or moderate in severity and can be treated with antihistamines if necessary.
Seek medical attention immediatelyif you experience the following symptoms, which may indicate the onset of an anaphylactic reaction:
- Sudden swelling of the face, lips, or throat
- Difficulty swallowing
- Difficulty breathing
- Hives
- Voice changes
- Worsening of existing asthma
- Nausea, abdominal pain, cramps, vomiting, or diarrhea
- Feeling of severe discomfort
Other possible side effects
Very common side effects (may affect more than 1 in 10 people)
- Reactions at the injection site, including: swelling, hives, discoloration, nodules, pain, bruising, hematoma, induration, inflammation, rash, feeling of warmth, lumps, redness, and/or itching
Common side effects (may affect up to 1 in 10 people)
- Severe allergic reactions
- Dizziness
- Conjunctivitis
- Wheezing
- Asthma symptoms, shortness of breath, bronchospasm, cough, or sneezing
- Throat irritation
- Nasal itching
- Feeling of discomfort in the nose, feeling of a blocked nose, or runny nose
- Abdominal pain, diarrhea, vomiting, nausea, heartburn
- Hives, itching, rash, redness of the skin
- Rash
- Flushes (sudden redness)
- Ear itching
- Discomfort
- Fatigue
- Chills
- Feeling of warmth
- Feeling of a foreign body in the throat
Uncommon side effects (may affect up to 1 in 100 people)
- Anaphylactic shock
- Swelling of the eyelids
- Swelling of the face
Other side effects with unknown frequency
- Feeling of pins and needles on the skin
- Feeling of rapid or irregular heartbeat
- Abnormal heart rate
- Bluish discoloration of the skin
- Low blood pressure
- Pallor
- Bronchospasm
- Feeling of constriction in the throat
- Swelling of the face or throat
- Joint swelling, joint pain
- Feeling of discomfort in the chest
- Increased hair growth at the injection site
Tell your doctor if you experience any side effects. This is important information for your doctor to determine the optimal dose of the medicine for you.
Reporting side effects
If you experience any side effects, including any possible side effects not listed in this leaflet, tell your doctor, pharmacist, or nurse.
Side effects can be reported directly to:
Department of Post-Marketing Surveillance of Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products,
Aleje Jerozolimskie 181 C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store ALUTARD SQ
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
Store in a refrigerator (2°C - 8°C). Do not freeze.
Store the vials in the outer packaging to protect from light.
After the first opening of the vial, the medicine can be used for 6 months if stored as recommended, i.e., in a refrigerator (2°C - 8°C); after this period, the medicine should be discarded.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What ALUTARD SQ contains
Animal-derived allergen extracts:
552 Horse dander
553 Dog dander
555 Cat dander
Basic treatment: 100 SQ-U/ml, 1000 SQ-U/ml, 10,000 SQ-U/ml, 100,000 SQ-U/ml
Maintenance treatment: 100,000 SQ-U/ml
suspension for injection
Excipients: aluminum hydroxide, hydrated, sodium chloride, sodium bicarbonate, phenol, sodium hydroxide – to adjust pH, water for injections.
What ALUTARD SQ looks like and contents of the pack
The basic treatment set contains 4 vials of 5 ml (from 100 SQ-U/ml to 100,000 SQ-U/ml).
The maintenance treatment set contains 1 vial of 5 ml (100,000 SQ-U/ml).
The vials are made of type I glass with chlorobutyl rubber stoppers and aluminum caps (different cap colors for each concentration: gray for 100 SQ-U/ml, green for 1000 SQ-U/ml, gold for 10,000 SQ-U/ml, red for 100,000 SQ-U/ml).
ALUTARD SQ should be administered by a qualified person (e.g., doctor, nurse).
It is recommended to store ALUTARD SQ in a medical facility.
Marketing authorization holder
ALK-Abelló A/S
Bøge Allé 6-8
DK-2970 Hørsholm
Denmark
Manufacturer
ALK-Abelló S.A.
Miguel Fleta 19
E-28037 Madrid
Spain
Date of last revision of the leaflet:
Information intended for healthcare professionals only:
Treatment with ALUTARD SQ should only be carried out by a doctor experienced in specific immunotherapy. The patient should be observed for a minimum of 30 minutes after each injection.
During storage, the appearance of a sediment and a clear liquid may be observed in the medicinal product. This is a normal phenomenon. The sediment may be white to light brown or greenish in color. Before use, the vial should be slowly turned upside down and back 10-20 times to obtain a homogeneous suspension. Before administration, the suspension should be inspected for the presence of solid particles. If solid particles are visible in the suspension, the product should be discarded.
ALUTARD SQ is administered subcutaneously. The product is injected either into the lateral distal part of the arm or dorsally into the proximal part of the forearm.
ALUTARD SQ should not be administered intravenously under any circumstances.
Avoid intravenous administration by performing careful aspiration before injecting the suspension. Aspiration should be repeated every 0.2 ml during injection of the product.
The injection must be administered slowly.
When using ALUTARD SQ, appropriate equipment and medications for the treatment of anaphylactic reactions should be available.
Since compatibility studies have not been conducted, this medicine should not be mixed with other medicines.
- Country of registration
- Prescription requiredNo
- Manufacturer
- ImporterALK-Abello S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Alutard SqDosage form: Lyophilizate, 12 SQ-HDMActive substance: house dust mitesManufacturer: ALK-Abello S.A.Prescription requiredDosage form: Tablets, 100 IRActive substance: house dust mitesManufacturer: Stallergenes S.A.S.Prescription requiredDosage form: Tablets, 100 IR+ 300 IRActive substance: house dust mitesManufacturer: Stallergenes S.A.S.Prescription required
Alternatives to Alutard Sq in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Alutard Sq in Ukraine
Alternative to Alutard Sq in Spain
Online doctors for Alutard Sq
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Alutard Sq – subject to medical assessment and local rules.














