How to use Alocutan
Package Leaflet: Information for the User
Alocutan, 20 mg/ml, scalp spray solution
Minoxidil
Read all of this leaflet carefully before using this medicine because it contains important information for you.
Always use this medicine exactly as described in this leaflet or as directed by your doctor or pharmacist.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your pharmacist.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
- If you do not feel better or worse, you should contact your doctor.
Contents of the pack
- 1. What is Alocutan and what is it used for
- 2. Important information before using Alocutan
- 3. How to use Alocutan
- 4. Possible side effects
- 5. How to store Alocutan
- 6. Contents of the pack and other information
1. What is Alocutan and what is it used for
Alocutan is indicated for the treatment of female pattern hair loss.
Alocutan is used to treat androgenetic alopecia (excessive hair loss)
in women (characteristic, diffuse, thinning of hair on the front-top area of the scalp in women). Treatment with Alocutan stimulates hair growth
and counteracts the progression of androgenetic alopecia.
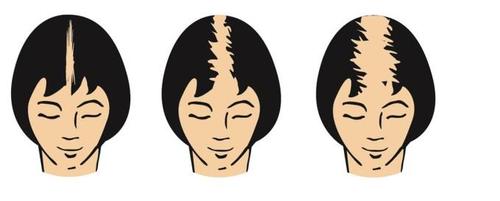
The onset of androgenetic alopecia in women is characterized by a decrease in hair thickness on the top and frontal area of the scalp, with preservation of the hairline. There is thinning of hair covering the central part of the scalp, but complete baldness is very rare.
The following illustrations show the stages of female pattern hair loss for which the efficacy of Alocutan has been demonstrated.
2. Important information before using Alocutan
When not to use Alocutan
- if you are hypersensitive to minoxidil or any of the other ingredients of this medicine (listed in section 6);
- if you are using other hair growth products on your scalp;
- if you are using any dressing or bandage on your scalp;
- if you have sudden and unexplained hair loss;
- if you are pregnant or breastfeeding;
- if you have any scalp disorders, including psoriasis (inflammatory skin disease with itching), sunburn, shaved scalp, or if your scalp has been damaged by burns or scarring, as hair follicles are not present.
Warnings and precautions
Before starting treatment with Alocutan, discuss it with your doctor or pharmacist.
You should rule out hormonal causes, underlying diseases, or malnutrition. In these cases, you will need special treatment.
Alocutan should only be applied to healthy, non-damaged scalp. Do not use Alocutan if the cause of hair loss is unknown, if you have hair loss after childbirth, if your scalp is infected or inflamed.
In case of systemic effects (systemic action) or severe skin reactions, discontinue use.
Alocutan is for external use on the scalp only. Do not use Alocutan on other parts of the body.
Do not use Alocutan:
- if you have symptoms of cardiovascular disorders or heart rhythm disorders;
- if you have high blood pressure;
- if you are taking medications for high blood pressure (antihypertensive drugs).
Stop using Alocutan and consult your doctor if:
- you experience low blood pressure,
- you experience any of the following symptoms: chest pain, rapid heartbeat, weakness, or dizziness, sudden unexplained weight gain, swelling of hands or feet, persistent redness or irritation of the scalp, or if you experience any new, unexpected symptoms (see "Using more than the recommended dose of Alocutan").
If the medicine comes into contact with areas other than the scalp, unwanted hair growth may occur in those areas.
Patients with very light hair have reported isolated cases of slight changes in hair color (slight discoloration of blonde hair) during concomitant use of the medicine and other hair care products or after bathing in heavily chlorinated water.
Accidental ingestion may lead to severe side effects from the cardiovascular system. Therefore, this medicine should be kept out of the reach of children.
There have been cases of excessive hair growth on the body of infants following skin contact with minoxidil application sites in patients (caregivers) using topical minoxidil. Hair growth returned to normal within a few months when the infant was no longer exposed to minoxidil. Be careful to avoid children coming into contact with areas of the body where minoxidil has been applied topically.
If excessive hair growth on the body is observed in a child during the use of topical minoxidil products, consult a doctor.
Avoid inhaling the medicine during application.
Children and adolescents
This medicine is not recommended for use in children and adolescents under 18 years of age, as the safety and efficacy have not been established in this age group.
Alocutan and other medicines
Tell your doctor or pharmacist about all the medicines you are taking, have recently taken, or might take.
There is no available information on interactions between Alocutan and other medicines.
Although not clinically confirmed, there is a theoretical possibility that absorbed minoxidil may enhance orthostatic hypotension (blood pressure drop when changing position from lying to standing) in patients taking concomitant peripheral vasodilators used to treat high blood pressure.
Alocutan should not be used with other dermatological medicines (external use products containing corticosteroids, retinoids, or ditranol) or with medicines that increase the absorption of the active substance through the skin.
Pregnancy and breastfeeding
There are only limited data on the use of Alocutan in pregnant women. Absorbed minoxidil may pass into breast milk. Therefore, Alocutan should not be used in pregnant or breastfeeding women.
Driving and using machines
This medicine may cause dizziness or hypotension (see section 4). If you experience symptoms, do not drive or operate machinery.
Alocutan contains propylene glycol (E 1520) and ethanol 96% (alcohol)
This medicine contains 199 mg of propylene glycol (E 1520) per ml.
This medicine contains 494 mg of alcohol (ethanol) per ml.
The medicine may cause burning of damaged skin.
Alcohol may cause burning and irritation of the eyes. In case of accidental contact with sensitive areas (eyes, damaged skin, mucous membranes), rinse the affected area with plenty of water.
Alocutan contains ethanol and propylene glycol, so repeated spraying on hair instead of the scalp may lead to increased dryness and/or stiffness of the hair.
3. How to use Alocutan
Always use this medicine exactly as described in this leaflet or as directed by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist.
Alocutan is for external use on dry scalp only. Apply Alocutan only to healthy, undamaged scalp and use it every time as described below. Do not use Alocutan on parts of the body other than the scalp.
Recommended dose
Unless your doctor has told you otherwise, use Alocutan as follows.
Apply 1 ml of Alocutan to the affected area of the scalp twice a day (morning and evening).
Do not exceed the daily dose of 2 ml, regardless of the size of the affected area.
Use in children and adolescents
Alocutan should not be used in children and adolescents under 18 years of age, as there are no available results from controlled clinical trials on its efficacy and safety in these age groups.
Method of administration
For cutaneous use (scalp).
Each pack of Alocutan contains two different applicators for the spray pump:
- a pre-mounted applicator for use on large areas,
- a separate applicator with an extended tip for use on small areas.
Both applicators can be exchanged by detaching one applicator and replacing it with the other.
To obtain a single dose of 1 ml of solution, 6 sprays of the aerosol are required.
Instructions for use/application
Spray the solution directly onto the scalp in the area of hair loss. To do this, press the pump 6 times. After each pump press, spread the liquid with your fingertips over the affected area, avoiding inhalation of the sprayed mist.
What to remember when using
After applying Alocutan, wash your hands carefully to avoid accidental contact with mucous membranes and eyes. After applying Alocutan, you can style your hair as usual.
However, do not wet your scalp for about 4 hours. This will prevent Alocutan from being washed off.
Duration of treatment
The onset and degree of hair regrowth vary among patients.
To see effects, treatment is usually started with application of the medicine twice a day for a period of 2 to 4 months. To maintain the effect, continued use is recommended without interruption, twice a day.
Using more Alocutan or using it more often will not lead to better results.
There is sufficient clinical experience regarding the possible therapeutic effect in case of treatment for up to 48 weeks.
If there is no improvement after 8 months, treatment should be stopped.
Information about increased hair loss
During treatment of hair follicles with minoxidil, the resting phase (telogen) in the hair growth cycle is shortened, and the transition to the growth phase (anagen) occurs faster. This stimulates the growth of new hair, causing old, inactive hair to fall out. As a result, there may initially be a feeling of increased hair loss.
In some patients, this reaction is observed within 2 to 6 weeks after starting treatment with minoxidil. However, do not be concerned, as this reaction is related to hair regrowth. This effect subsides within a few weeks and may be considered the first sign of minoxidil's effect.
If you feel that Alocutan is too strong or too weak, you should contact your doctor or pharmacist.
Using more than the recommended dose of Alocutan
Using Alocutan in doses higher than recommended and on relatively large areas of the body or on other areas of the body than the scalp may lead to increased systemic absorption of minoxidil. To date, no cases of poisoning due to topical application of a minoxidil solution have been reported.
Given the concentration of the active substance in Alocutan, accidental ingestion may lead to systemic effects, such as those that occur after ingestion of the active substance, e.g., in tablet form. This may lead to the following side effects: rapid heartbeat, low blood pressure, fluid retention, and consequently sudden weight gain, dizziness.
In case of accidental ingestion of the medicine or if you experience symptoms of overdose, inform your doctor immediately so that they can decide what to do next. Bring the package of the medicine with you to inform the doctor which active substance was taken.
Missing a dose of Alocutan
Do not take a double dose to make up for a forgotten dose. Continue treatment, using the recommended dose. Making up for a forgotten dose will not provide any benefit and may lead to side effects.
Stopping use of Alocutan
Continue treatment to improve and maintain hair growth. Otherwise, hair loss will recur.
If treatment with Alocutan is stopped, hair loss will recur within 3 to 4 months, and the condition will return to what it was before treatment.
If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Seek immediate medical attention if you experience any of the following symptoms - you may need urgent medical attention.
- Swelling of the face, lips, or throat, which makes swallowing or breathing difficult. This may be a sign of a severe allergic reaction (frequency not known, cannot be estimated from the available data).
- Generalized redness of the skin (frequency not known, cannot be estimated from the available data).
- Generalized itching (frequency not known, cannot be estimated from the available data).
- Throat constriction (frequency not known, cannot be estimated from the available data).
Very common(may affect more than 1 in 10 people):
- headache.
Common(may affect up to 1 in 10 people):
- itching,
- excessive hair growth outside the scalp (including hair growth on the face in women),
- skin inflammatory reactions (including acne-like rash and skin rash),
- shortness of breath, difficulty breathing,
- swelling of hands and feet,
- weight gain,
- high blood pressure,
- irritation of the scalp, such as pricking, burning, dryness, itching, flaking, folliculitis.
Uncommon(may affect up to 1 in 100 people):
- dizziness, nausea.
Frequency not known(frequency cannot be estimated from the available data):
- contact dermatitis (allergic skin inflammation),
- depressive mood,
- eye irritation,
- rapid heartbeat, palpitations, low blood pressure,
- vomiting,
- local reactions, including ears and face, such as itching, skin irritation, pain, redness, swelling, dry skin, inflammatory rash, dermatitis, blisters, bleeding, and ulceration,
- temporary hair loss, changes in hair color, changes in hair texture,
- chest pain.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor, pharmacist, or nurse. You can also report side effects directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
You can also report side effects to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Alocutan
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and bottle after EXP. The expiry date refers to the last day of that month.
Do not freeze.
Contains flammable ethanol. Keep away from heat sources or open flames.
Shelf life after first opening
Shelf life after first opening of the bottle: 6 weeks.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Alocutan contains
- The active substance is minoxidil. 1 ml of solution contains 20 mg of minoxidil.
- The other ingredients are ethanol 96% (V/V), propylene glycol (E 1520), purified water.
What Alocutan looks like and contents of the pack
Alocutan is a clear, colorless to light yellow solution available in the following packs: 1 x 60 ml and 3 x 60 ml solution for cutaneous use in white HDPE bottles with a spray pump, in a cardboard box.
Each pack contains two applicators: one pre-mounted and one with an extended tip.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
SUN-FARM Sp. z o.o.
ul. Dolna 21
05-092 Łomianki
Manufacturer
mibe GmbH Arzneimittel
Münchener Straße 15
06796 Brehna
Germany
SUN-FARM Sp. z o.o.
ul. Dolna 21
05-092 Łomianki
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria
Alocutan 20 mg/ml Lösung zur Anwendung auf der Haut
Germany
Minoxicutan Frauen 20 mg/ml Lösung zur Anwendung auf der Haut (Kopfhaut)
Poland
Alocutan
Date of last revision of the leaflet:09.2024
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- Importermibe GmbH Arzneimittel Sun-Farm Sp. z o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AlocutanDosage form: Solution, 20 mg/mlActive substance: minoxidilManufacturer: Industrial Farmaceutica Cantabria, S.A.Prescription not requiredDosage form: Solution, 50 mg/mlActive substance: minoxidilManufacturer: Industrial Farmaceutica Cantabria, S.A.Prescription not requiredDosage form: Aerosol, 50 mg/mlActive substance: minoxidilManufacturer: mibe GmbH Arzneimittel Sun-Farm Sp. z o.o.Prescription not required
Alternatives to Alocutan in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Alocutan in Ukraine
Alternative to Alocutan in Spain
Online doctors for Alocutan
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Alocutan – subject to medical assessment and local rules.