

Adrenalina Vzf 0,1%

Ask a doctor about a prescription for Adrenalina Vzf 0,1%

How to use Adrenalina Vzf 0,1%
Leaflet attached to the packaging: patient information
ADRENALINA WZF 0.1%, 1 mg/ml, solution for injection
Adrenalinum
Please read carefully the contents of the leaflet before using the medicine, as it contains important information for the patient.
- Please keep this leaflet, so that you can read it again if necessary.
- In case of any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Adrenalina WZF 0.1% and what is it used for
- 2. Important information before using Adrenalina WZF 0.1%
- 3. How to use Adrenalina WZF 0.1%
- 4. Possible side effects
- 5. How to store Adrenalina WZF 0.1%
- 6. Contents of the packaging and other information
1. What is Adrenalina WZF 0.1% and what is it used for
The active substance of the medicine is adrenaline. Adrenaline constricts blood vessels, accelerates heart rate, and dilates airways. The medicine is administered by a doctor in life-threatening situations in the following cases:
- in sudden cardiac arrest - cardiopulmonary resuscitation (i.e., restoring life by performing procedures that temporarily replace heart and lung function);
- in anaphylactic shock and other severe allergic reactions;
- in an asthma attack - to interrupt bronchospasm;
- in severe bradycardia (slow heart rate);
- in cardiogenic shock - as a vasoconstrictor (a medicine that constricts blood vessels).
2. Important information before using Adrenalina WZF 0.1%
When not to use Adrenalina WZF 0.1%
- if the patient is allergic to adrenaline or any other ingredient of the medicine (listed in section 6), although in life-threatening situations, there are no absolute contraindications to the use of the medicine.
Warnings and precautions
Before starting treatment with Adrenalina WZF 0.1%, the patient should discuss it with their doctor or pharmacist.
Particular caution should be exercised when using Adrenalina WZF 0.1% in the following cases:
- heart disease (e.g., coronary artery disease, arrhythmias, right ventricular hypertrophy);
- hyperthyroidism;
- hypertension;
- diabetes;
- increased intraocular pressure;
- pheochromocytoma;
- prostatic hyperplasia (leading to urinary retention);
- increased blood calcium levels;
- decreased blood potassium levels;
- severe renal failure;
- in elderly patients.
Adrenalina WZF 0.1% and other medicines
The patient should inform their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
- Adrenaline can be used with other medicines, but its effects may be enhanced by:
- medicines used to treat depression (e.g., venlafaxine, milnacipran, and monoamine oxidase inhibitors);
- medicines used to treat Parkinson's disease (e.g., entacapone, a medicine that blocks the enzyme methylation; levodopa);
- thyroid hormones;
- theophylline (an anti-asthmatic medicine);
- oxytocin (a hormonal medicine used in obstetrics);
- parasympatholytics (e.g., atropine);
- certain antihistamines, such as diphenhydramine, chlorpheniramine;
- sympathomimetic medicines (e.g., bronchodilator inhalers).
- Alcohol enhances the effects of adrenaline.
- When administering adrenaline and medicines such as propranolol, sotalol (and other non-selective β-adrenoreceptor blockers), severe hypertension and bradycardia may occur.
- The medicine should be used with caution in patients taking digitalis glycosides, quinidine, chloroform-based anesthetics, as these medicines may increase the risk of arrhythmias.
- The increase in blood pressure caused by adrenaline can be counteracted by rapidly acting vasodilators or α-adrenergic receptor blockers.
- The effects of adrenaline can be reversed by β-adrenergic receptor blockers, especially non-selective ones (e.g., propranolol).
- Adrenaline inhibits insulin secretion, so in diabetic patients, it may be necessary to increase the dose of insulin or other diabetes medicines.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
The medicine can be used in pregnant women if, in the doctor's opinion, the benefit to the mother outweighs the potential risk to the fetus.
Due to the minimal penetration of adrenaline into breast milk, it is unlikely to affect the breastfed child.
Driving and operating machinery
After using adrenaline, the patient should not drive or operate machinery until the disturbances that were the indication for administration of the medicine have subsided.
Adrenalina WZF 0.1% contains sodium metabisulfite (E 223) and sodium
Adrenalina WZF 0.1% contains sodium metabisulfite (E 223) - a medicine that can rarely cause severe hypersensitivity reactions and bronchospasm.
The medicine contains 3.39 mg of sodium (the main component of table salt) in each ampoule (1 ml of solution).
This corresponds to 0.17% of the maximum recommended daily dose of sodium in the diet for adults.
The medicine may be diluted - see below "Information intended exclusively for healthcare professionals". The sodium content from the diluent should be taken into account when calculating the total sodium content in the prepared dilution of the medicine. To obtain accurate information about the sodium content in the solution used for dilution, you should consult the leaflet of the diluent used.
3. How to use Adrenalina WZF 0.1%
This medicine should always be used as directed by a doctor or pharmacist. In case of doubts, the patient should consult their doctor.
Adrenalina WZF 0.1% is administered by a doctor subcutaneously, intramuscularly, or intravenously.
The dose of the medicine is adjusted by the doctor according to the patient's condition. Detailed data are provided
in the section "Information intended exclusively for healthcare professionals".
Using a higher dose of Adrenalina WZF 0.1% than recommended
In case of administration of a large dose of the medicine or accidental injection into a vessel, there may be a sudden increase in blood pressure, vasoconstriction, and cardiac stimulation. There may be bradycardia or tachycardia, arrhythmias, or difficulty breathing. The healthcare staff will take appropriate action.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The occurrence of side effects depends on the patient's sensitivity to adrenaline and the dose administered.
Common side effects (occurring in less than 1 in 10 people), even after administration of small doses of adrenaline, include: palpitations, tachycardia, sweating, nausea, vomiting, difficulty breathing, pallor, dizziness, weakness, tremors, headache, nervousness, anxiety, and a feeling of coldness in the hands and feet.
Rarely (occurring in less than 1 in 100 people), the following have been reported:
hallucinations, fainting, increased blood sugar levels, decreased blood potassium levels, metabolic acidosis, dilated pupils, difficulty urinating, including urinary retention, and muscle tremors.
Side effects that have occurred after administration of large doses of adrenaline or in sensitive individuals: arrhythmias (atrial fibrillation or cardiac arrest), sudden increase in blood pressure (sometimes leading to stroke), and vasoconstriction (e.g., in the skin, mucous membranes, kidneys).
The medicine contains sodium metabisulfite, which can cause allergic reactions, including anaphylactic symptoms and bronchospasm in sensitive individuals, especially those with asthma.
Reporting side effects
If any side effects occur, including any not listed in this leaflet, the patient should inform their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. Storage of Adrenalina WZF 0.1%
Store in a refrigerator (2°C - 8°C). Store the ampoules in the outer packaging to protect from light. Do not freeze.
The medicine can be stored for 6 months at a temperature below 25°C.
The medicine should be kept out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton. The expiry date refers to the last day of the month.
The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
- The active substance of the medicine is adrenaline. Each ml of solution for injection contains 1 mg of adrenaline (as adrenaline tartrate).
- The other ingredients are: sodium metabisulfite (E223), sodium chloride, water for injections.
What Adrenalina WZF 0.1% looks like and what the packaging contains
Adrenalina WZF 0.1% is a colorless or almost colorless, clear liquid.
The packaging consists of 10 ampoules of 1 ml each in a cardboard box.
Marketing authorization holder and manufacturer
Zakłady Farmaceutyczne POLPHARMA S.A.
ul. Pelplińska 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Date of last revision of the leaflet:December 2024
Information intended exclusively for healthcare professionals:
ADRENALINA WZF 0.1%, 1 mg/ml, solution for injection
Adrenalinum
Instructions for opening the ampoule
Before opening the ampoule, make sure that the entire solution is in the lower part of the ampoule.
You can gently shake the ampoule or tap it with your finger to facilitate the flow of the solution.
A colored dot is placed on each ampoule (see Figure 1) as a mark indicating the location of the break point below it.
- To open the ampoule, hold it vertically, with both hands, with the colored dot facing you - see Figure 2. The upper part of the ampoule should be grasped in such a way that the thumb is above the colored dot.
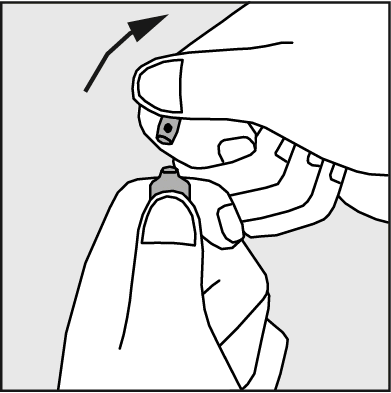
- Press in the direction of the arrow shown in Figure 3.
Ampoules are intended for single use only and should be opened immediately before use. Any remaining contents of the unused product should be disposed of in accordance with applicable regulations.
Figure 1.
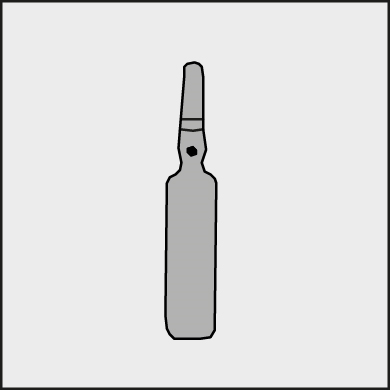
Figure 2.
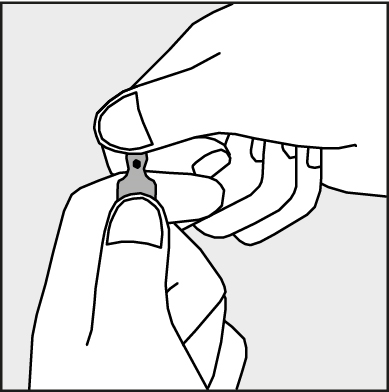
Figure 3.
Adrenalina WZF 0.1% can be administered subcutaneously, intramuscularly, or intravenously.
In the initial treatment of anaphylaxis, the intramuscular route of administration of adrenaline is recommended. The intravenous route of administration of adrenaline is intended for use in intensive care units or emergency departments. The adrenaline solution with a concentration of 1 mg/ml (1:1000) is not suitable for intravenous use. If adrenaline in a solution of 1:10,000 (0,1 mg/ml) in the form of a solution for injection is not available, the 1:1000 solution must be diluted to a 1:10,000 solution before intravenous administration - preparation of the dilution, see below. When administering adrenaline intravenously, extreme caution should be exercised, and the intravenous route is reserved exclusively for specialists experienced in intravenous administration of adrenaline.
Adults
Sudden cardiac arrest - cardiopulmonary resuscitation
- Intravenously 1 mg of adrenaline after 10-fold dilution or without dilution, followed by a bolus of 10 ml of 0.9% NaCl solution. If necessary, doses can be repeated every 3 to 5 minutes.
After restoration of spontaneous circulation, if the use of adrenaline is still necessary, it can be administered slowly intravenously in small doses (boluses of 50 to 100 µg), until the desired effect is achieved.
Anaphylactic shock and other severe allergic reactions
- Intramuscularly or subcutaneously 0.3 to 0.5 mg. In severe cases, 1 mg in a single dose. In anaphylactic shock, the intramuscular route is preferred. If necessary, doses can be repeated every 10 to 15 minutes, taking into account blood pressure, heart rate, and respiratory function.
- Slowly intravenously 0.3 to 1 mg after dilution (10-fold or greater). Intravenous administration is reserved exclusively for patients with severe, life-threatening shock or in special situations, e.g., during general anesthesia.
Asthma attack - to interrupt bronchospasm
- Subcutaneously or intramuscularly 0.3 mg. Doses can be repeated three times every 20 minutes.
Severe bradycardia
- If atropine is ineffective, adrenaline should be considered, administered as an intravenous infusion at a rate of 2 to 10 µg/min. The medicine should be administered until the desired effect is achieved.
Cardiogenic shock - as a vasoconstrictor
- As a second-line medicine - intravenously 0.05 to 0.5 µg/kg body weight/min.
Children
Sudden cardiac arrest - cardiopulmonary resuscitation
- Intravenously 10 µg/kg body weight. If necessary, doses can be repeated every 3 to 5 minutes.
After restoration of spontaneous circulation, if the use of adrenaline is still necessary, it can be administered as an intravenous infusion at a rate of 0.05 to 1.0 µg/kg body weight/min. The infusion should be administered until the desired effect is achieved. In children, there are significant individual differences in response to adrenaline.
Anaphylactic shock and other severe allergic reactions
Intramuscularly or subcutaneously 10 µg/kg body weight, up to a maximum single dose of 0.5 mg.
In anaphylactic shock, the intramuscular route is preferred.
If necessary, the doses given above can be repeated several times, with an interval of 5 to 15 minutes, taking into account blood pressure, heart rate, and respiratory function.
It is recommended to use small-volume syringes.
Slowly intravenously 10 µg/kg body weight after dilution (10-fold or greater). Intravenous administration is reserved exclusively for patients with severe, life-threatening shock or in special situations, e.g., during general anesthesia.
Asthma attack - to interrupt bronchospasm
| Age | Dose of adrenaline 1 mg/ml (1:1000 solution) |
| Over 12 years | 0.5 mg intramuscularly (0.5 ml of 1:1000 solution) |
| 6 to 12 years | 0.3 mg intramuscularly (0.3 ml of 1:1000 solution) |
| 6 months to 6 years | 0.15 mg intramuscularly (0.15 ml of 1:1000 solution) |
| Under 6 months | 0.01 mg/kg body weight intramuscularly (0.01 ml/kg body weight of 1:1000 solution) |
- Subcutaneously or intramuscularly 10 µg/kg body weight, up to a maximum single dose of 0.5 mg. Doses can be repeated twice every 20 minutes, and then every 4 hours if necessary.
Severe bradycardia
- Intravenous infusion at a rate of 0.05 to 1.0 µg/kg body weight/min.
Cardiogenic shock - as a vasoconstrictor
- As a second-line medicine, intravenously at a rate of 0.05 to 1.0 µg/kg body weight/min.
IN LIFE-THREATENING SITUATIONS!
Adults
- If it is impossible to gain access to a vein, the medicine can be administered intrathecally (intraosseously) in doses used intravenously.
- Intratracheally 3 mg after dilution. For this purpose, 3 ml of adrenaline should be diluted in 10 ml of 0.9% NaCl solution or water for injections and administered through an endotracheal tube. Dilution with water for injections results in better absorption of the medicine.
Children
- If it is impossible to gain access to a vein, the medicine can be administered intrathecally (intraosseously) in doses used intravenously or intratracheally in a dose of 100 µg/kg body weight after prior dilution in 5 ml of 0.9% NaCl solution.
WARNING:Adrenaline undergoes accelerated degradation in an alkaline environment. The medicine should not be mixed with bicarbonates.
The medicine can be diluted in the following infusion solutions: 0.9% NaCl solution, water for injections, or 5% glucose solution. Solutions should be prepared immediately before administration.
Adrenaline is most commonly used in the following concentrations:
1:1000 (1 mg of adrenaline/1 ml of solution) - ready-to-use medicine,
1:10,000 (1 mg of adrenaline/10 ml of solution),
1:100,000 (1 mg of adrenaline/100 ml of solution).
See also the section "Adrenalina WZF 0.1% contains sodium metabisulfite (E 223) and sodium".
The sodium content from the diluent should be taken into account when calculating the total sodium content in the prepared dilution of the medicine. To obtain accurate information about the sodium content in the solution used for dilution, you should consult the leaflet of the diluent used.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterZakłady Farmaceutyczne POLPHARMA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Adrenalina Vzf 0,1%Dosage form: Solution, 1 mg/10 mlActive substance: epinephrineManufacturer: Laboratoire AguettantPrescription requiredDosage form: Solution, 300 mcg/0.3 mlActive substance: epinephrineManufacturer: Warszawskie Zakłady Farmaceutyczne POLFA S.A.Prescription requiredDosage form: Solution, 0.15 mg/doseActive substance: epinephrineManufacturer: Meda Pharma GmbH & Co. KGPrescription required
Alternatives to Adrenalina Vzf 0,1% in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Adrenalina Vzf 0,1% in Ukraine
Alternative to Adrenalina Vzf 0,1% in Spain
Online doctors for Adrenalina Vzf 0,1%
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Adrenalina Vzf 0,1% – subject to medical assessment and local rules.










