
VEROLAX NIÑOS RECTAL SOLUTION

How to use VEROLAX NIÑOS RECTAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
VEROLAX CHILDREN Rectal Solution
Glycerol
Read the package leaflet carefully because it contains important information for you.
This medication can be obtained without a prescription. Nevertheless, to obtain the best results, it should be used properly.
- Keep this package leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If symptoms worsen or persist after 7 days, you should consult a doctor.
- If you consider that any of the adverse effects you are suffering from is serious or if you notice any adverse effect not mentioned in this package leaflet, inform your doctor or pharmacist.
Contents of the Package Leaflet
- What is VEROLAX and what is it used for
- Before using VEROLAX
- How to use VEROLAX
- Possible side effects
- Storage of VEROLAX
- Additional information
1. What is VEROLAX and what is it used for
Glycerol, the active ingredient of this medication, is a laxative that is administered rectally. The laxative effect is achieved by glycerol's ability to soften the stool, which, along with a mild local irritating action, stimulates intestinal movements.
It is indicated for the local symptomatic relief of transient and occasional constipation in children between 2 and 12 years old.
2. Before using VEROLAX
- If you are allergic (hypersensitive) to glycerol or any of the other components of this medication.
- If you have any anorectal disease, hemorrhagic rectocolitis (a type of chronic inflammation of the intestine), and inflamed hemorrhoids.
- If you have cramps, colic, nausea, vomiting, or other signs of appendicitis, intestinal obstruction, acute inflammatory intestinal diseases, or any situation of abdominal pain without knowing the cause.
- Children under 2 years old.
Be careful with VEROLAX
In case of the appearance of blood in stool, irritation, pain, or no improvement, you should interrupt treatment and consult a doctor.
Do not use this medication for more than 7 consecutive days, unless your doctor indicates otherwise.
This medication will only be used under strict medical control in patients with severe diseases, especially cardiovascular ones (related to the heart or blood vessels).
Use of other medications
Inform your doctor or pharmacist if you are using or have recently used other medications, including those obtained without a prescription.
Use in children
It should not be administered to children under 2 years old.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before using any medication.
Driving and using machines
The use of this medication does not affect the ability to drive and/or use machines.
3. How to use VEROLAX
Follow the administration instructions of VEROLAX exactly as indicated by your doctor.
Consult your doctor or pharmacist if you have any doubts.
The normal dose is:
Children from 2 to 12 years old: 1 single-dose container per day.
This medication is administered rectally.
Instructions for the correct administration of the medication:
- Open the applicator by holding it by the neck and twisting the cap, facilitating the breaking of the seal.
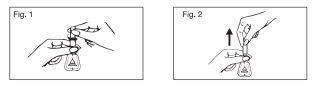
- Press lightly so that a few drops of the medication moisten the end of the cannula, in order to facilitate its introduction into the rectum.
- Insert the cannula of the applicator into the rectum smoothly and slowly.
- Insert the contents of the applicator by pressing on the walls of the bellows and without stopping to press, gently remove it once the application is finished.
Each applicator is loaded with an excess of product, which remains in the applicator after use, so the amount released during application is the complete dose, and it is not necessary to empty it completely, which facilitates its handling. It should be discarded after use.
If you notice resistance during administration, you should interrupt it, as it may be harmful and damaging.
Do not use the medication for more than 7 consecutive days. If symptoms do not improve, you should suspend treatment and consult a doctor.
If you use more VEROLAX than you should
It is unlikely that intoxication will occur due to its use.
The abusive and prolonged use of this medication can lead to an irritable colon syndrome (symptoms or discomfort such as alternation of constipation and diarrhea, intestinal spasms, bloating, nausea, and gases).
In case of overdose or accidental ingestion, consult your doctor, pharmacist, or call the Toxicology Information Service, phone 91.562.04.20, indicating the medication and the amount ingested.
4. Possible side effects
Like all medications, VEROLAX can cause side effects, although not everyone will experience them.
During the period of use of VEROLAX rectal solution as a laxative, the following side effects have been observed, whose frequency has not been established with precision:
itching, pain, and irritation of the anus.
Very rarely, in allergic patients, hypersensitivity reactions to chamomile (e.g., contact dermatitis) have occurred. After internal use, these could be serious and could be: swelling of the face, lips, mouth, tongue, or throat that causes difficulty swallowing or breathing.
If you consider that any of the side effects you are suffering from is serious or if you notice any side effect not mentioned in this package leaflet, inform your doctor or pharmacist.
5. Storage of VEROLAX
Keep out of the reach and sight of children.
No special storage conditions are required.
Do not use VEROLAX after the expiration date that appears on the container, after CAD. The expiration date is the last day of the month indicated.
Medications should not be thrown away in drains or trash. Ask your pharmacist how to dispose of the containers and medications you no longer need. This will help protect the environment.
6. ADDITIONAL INFORMATION
Composition of VEROLAX
- The active ingredient is glycerol. Each single-dose container with 2.5 ml of rectal solution contains 1.8 ml of glycerol.
- The other components (excipients) are: fluid extract of Malva sylvestris, fluid extract of Matricaria chamomilla, wheat starch, and purified water.
Appearance of the product and container contents
VEROLAX is presented as a light brown rectal solution.
The medication is packaged in a single-dose container in the form of a 2.5 ml bellows, equipped with a plastic cannula and a closing cap. These are packaged in a box containing 6 single-dose containers.
Marketing authorization holder
ANGELINI PHARMA ESPAÑA, S.L.
c/ Antonio Machado, 78-80
3rd floor, module A-Australia Building
08840 Viladecans, Barcelona (Spain)
Manufacturer
A.C.R.A.F. SpA
Via Vecchia del Pinocchio, 22
60131 Ancona (Italy)
This package leaflet was approved in May 2011.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VEROLAX NIÑOS RECTAL SOLUTIONDosage form: RECTAL LIQUID, 6.14 ml glycerolActive substance: glycerolManufacturer: Casen Recordati S.L.Prescription not requiredDosage form: RECTAL LIQUID, 6.75 g/single-dose tubeActive substance: glycerolManufacturer: Laboratorios Cinfa S.A.Prescription not requiredDosage form: RECTAL LIQUID, 5.4 ml of glycerol/single-dose containerActive substance: glycerolManufacturer: Lainco S.A.Prescription not required
Online doctors for VEROLAX NIÑOS RECTAL SOLUTION
Discuss questions about VEROLAX NIÑOS RECTAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















