BYLVAY 200 MICROGRAMOS CAPSULAS DURAS


Cómo usar BYLVAY 200 MICROGRAMOS CAPSULAS DURAS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Bylvay 200 microgramos cápsulas duras
Bylvay 400 microgramos cápsulas duras
Bylvay 600 microgramos cápsulas duras
Bylvay 1200 microgramos cápsulas duras
odevixibat
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto (ver sección 4).
Contenido del prospecto
- Qué es Bylvay y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Bylvay
- Cómo tomar Bylvay
- Posibles efectos adversos
- Conservación de Bylvay
- Contenido del envase e información adicional
1. Qué es Bylvay y para qué se utiliza
Bylvay contiene el principio activo odevixibat. Odevixibat es un fármaco que aumenta la eliminación del organismo de unas sustancias llamadas ácidos biliares. Los ácidos biliares son componentes del líquido digestivo denominado bilis, que es producido por el hígado y secretado al intestino. Odevixibat bloquea el mecanismo que normalmente los reabsorbe del intestino después de haber hecho su trabajo. De este modo se pueden eliminar del cuerpo a través de las heces.
Bylvay se utiliza para tratar la colestasis intrahepática familiar progresiva (CIFP) en pacientes de 6 meses o más. La CIFP es una enfermedad hepática causada por la acumulación de ácidos biliares (colestasis) que empeora con el tiempo y que se acompaña a menudo de fuertes picores.
2. Qué necesita saber antes de empezar a tomar Bylvay
No tome Bylvay
- si es alérgico a odevixibat o a alguno de los demás componentes de este medicamento (incluidos en la sección 6)
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Bylvay:
- si se le ha diagnosticado una ausencia completa o una falta de función de la proteína de la bomba de exportación de sales biliares;
- si presenta disfunción hepática grave;
- si presenta una reducción de la motilidad estomacal o intestinal o una reducción de la circulación de ácidos biliares entre el hígado, la bilis y el intestino delgado debido a medicamentos, intervenciones quirúrgicas o enfermedades distintas de la CIFP, ya que en estos casos puede disminuir el efecto del odevixibat.
Consulte a su médico si presenta diarrea mientras toma Bylvay. Se recomienda beber suficiente líquido a los pacientes con diarrea para evitar la deshidratación.
Durante el tratamiento con Bylvay se pueden observar niveles elevados de enzimas hepáticas en las pruebas de función hepática. Antes de empezar a tomar Bylvay, su médico medirá su función hepática para comprobar el funcionamiento de su hígado. Su médico realizará controles regulares para monitorizar su función hepática.
Antes y durante el tratamiento, su médico puede controlar también sus niveles sanguíneos de vitamina A, D y E y su INR (ratio normalizada internacional, que mide su riesgo de sangrado).
Niños
No se recomienda el uso de Bylvay en bebés menores de 6 meses, ya que no se sabe si el medicamento es seguro y eficaz en este grupo de edad.
Uso de Bylvay con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
El tratamiento con odevixibat puede afectar a la absorción de vitaminas liposolubles, como las vitaminas A, D y E, y de algunos medicamentos.
Embarazo y lactancia
Si está embarazada o en período de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
No se recomienda utilizar Bylvay durante el embarazo ni en mujeres en edad fértil que no estén utilizando métodos anticonceptivos.
No se sabe si odevixibat puede pasar a la leche materna y afectar al bebé. Su médico la ayudará a decidir si es mejor interrumpir la lactancia o evitar el tratamiento con Bylvay, teniendo en cuenta el beneficio de la lactancia para el bebé y el de Bylvay para la madre.
Conducción y uso de máquinas
La influencia de Bylvay sobre la capacidad para conducir y utilizar máquinas es nula o insignificante.
3. Cómo tomar Bylvay
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
El tratamiento debe ser iniciado y supervisado por un médico con experiencia en el tratamiento de la hepatopatía progresiva con reducción del flujo biliar.
La dosis de Bylvay depende de su peso. Su médico calculará el número correcto de cápsulas que debe tomar y su concentración.
La dosis recomendada es
- 40 microgramos de odevixibat por kilogramo de peso corporal una vez al día
- Si el medicamento no funciona suficientemente bien después de 3 meses, su médico puede aumentar la dosis a 120 microgramos de odevixibat por kilogramo de peso corporal (hasta un máximo de 7 200 microgramos una vez al día).
No se recomiendan dosis diferentes en adultos.
Modo de empleo
Tome las cápsulas una vez al día por la mañana, con o sin alimentos.
Todas las cápsulas se pueden tragar enteras con un vaso de agua o bien abrirse y espolvorear su contenido sobre algún alimento o en un líquido adecuado para la edad (por ejemplo, leche materna, leche artificial o agua).
Las cápsulas más grandes, de 200 y 600 microgramos, están diseñadas para abrirse y espolvorear el contenido sobre algún alimento o en un líquido adecuado para la edad, pero se pueden tragar enteras. Las cápsulas más pequeñas, de 400 y 1 200 microgramos, están diseñadas para tragarse enteras, pero se pueden abrir y espolvorear el contenido sobre algún alimento o en un líquido adecuado para la edad.
Encontrará instrucciones detalladas acerca de cómo abrir las cápsulas y espolvorear el contenido sobre algún alimento o en un líquido, al final de este prospecto.
Si el medicamento no mejora su enfermedad después de 6 meses de tratamiento diario continuo, su médico le recomendará un tratamiento alternativo.
Si toma más Bylvay del que debe
Informe a su médico si cree que ha tomado demasiado Bylvay.
Los posibles síntomas de una sobredosis son diarrea y problemas de estómago e intestino.
Si olvidó tomar Bylvay
No tome una dosis doble para compensar la dosis olvidada. Tome la siguiente dosis a la hora habitual.
Si interrumpe el tratamiento con Bylvay
No deje de tomar Bylvay sin hablar antes con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos pueden aparecer con las siguientes frecuencias:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- diarrea, incluida dirarrea con heces sanguinolentas, heces blandas
- vómitos
- dolor abdominal (de barriga)
Frecuentes(pueden afectar hasta a 1 de cada 10 personas)
- aumento de tamaño del hígado
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Bylvay
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y el frasco después de EXP. La fecha de caducidad es el último día del mes que se indica.
Conservar en el embalaje original para protegerlo de la luz. No conservar a temperatura superior a 25 °C.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Bylvay
- El principio activo es el odevixibat.
Cada cápsula dura de Bylvay 200 microgramos contiene 200 microgramos de odevixibat (como sesquihidrato).
Cada cápsula dura de Bylvay 400 microgramos contiene 400 microgramos de odevixibat (como sesquihidrato).
Cada cápsula dura de Bylvay 600 microgramos contiene 600 microgramos de odevixibat (como sesquihidrato).
Cada cápsula dura de Bylvay 1 200 microgramos contiene 1 200 microgramos de odevixibat (como sesquihidrato).
Los demás componentes son:
- Contenido de la cápsula
Celulosa microcristalina
Hipromelosa
Cubierta de la cápsula
Bylvay 200 microgramos y 600 microgramos cápsulas duras
Hipromelosa
Dióxido de titanio (E171)
Óxido de hierro amarillo (E172)
Bylvay 400 microgramos y 1 200 microgramos cápsulas duras
Hipromelosa
Dióxido de titanio (E171)
Óxido de hierro amarillo (E172)
Óxido de hierro rojo (E172)
Tinta de impresión
Goma laca
Propilenglicol
Óxido de hierro negro (E172)
Aspecto del producto y contenido del envase
Bylvay 200 microgramos cápsulas duras:
Cápsulas de tamaño 0 (21,7 mm × 7,64 mm) con tapa opaca de color marfil y cuerpo opaco de color blanco; con la inscripción «A200» en tinta negra.
Bylvay 400 microgramos cápsulas duras:
Cápsulas de tamaño 3 (15,9 mm × 5,82 mm) con tapa opaca de color naranja y cuerpo opaco de color blanco; con la inscripción «A400» en tinta negra.
Bylvay 600 microgramos cápsulas duras:
Cápsulas de tamaño 0 (21,7 mm × 7,64 mm) con tapa y cuerpo opacos de color marfil; con la inscripción «A600» en tinta negra.
Bylvay 1 200 microgramos cápsulas duras:
Cápsulas de tamaño 3 (15,9 mm × 5,82 mm) con tapa y cuerpo opacos de color naranja; con la inscripción «A1200» en tinta negra.
Las cápsulas duras de Bylvay se acondicionan en un frasco de plástico con cierre de polipropileno, con precinto de seguridad y a prueba de niños. Tamaño del envase: 30 cápsulas duras.
Titular de la autorización de comercialización
Ipsen Pharma
70 rue Balard
75015 París
Francia
Responsable de la fabricación
Almac Pharma Services Limited
Seagoe Industrial Estate
Portadown, Craigavon
County Armagh
BT63 5UA
Reino Unido (Irlanda del Norte)
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien/Luxembourg/ Luxemburg Ipsen NV België/Belgique/Belgien Tél/Tel: +32 9 243 96 00 | Italia Ipsen SpA Tel: + 39 02 39 22 41 |
| Latvija Ipsen Pharma representative office Tel: + 371 67622233 |
| Lietuva Ipsen Pharma SAS Lietuvos filialas Tel: +370 700 33305 |
Danmark, Norge, Suomi/Finland, Sverige, Ísland Institut Produits Synthèse (IPSEN) AB Sverige/Ruotsi/Svíþjóð Tlf/Puh/Tel/Sími: +46 8 451 60 00 | Magyarország IPSEN Pharma Hungary Kft. Tel.: + 36 1 555 5930 |
Deutschland, Österreich Ipsen Pharma GmbH Deutschland Tel: +49 89 2620 432 89 | Nederland Ipsen Farmaceutica B.V. Tel: +31 (0) 23 554 1600 |
Eesti Centralpharma Communications OÜ Tel: +372 60 15 540 | Polska Ipsen Poland Sp. z o.o. Tel.: + 48 22 653 68 00 |
Ελλáδα, Κúπρος, Malta Ipsen Μονοπρ?σωπη EΠΕ Ελλáδα Τηλ: +30 210 984 3324 | Portugal Ipsen Portugal - Produtos Farmacêuticos S.A. Tel: + 351 21 412 3550 |
España Ipsen Pharma, S.A.U. Tel: +34 936 858 100 | România Ipsen Pharma România SRL Tel: + 40 21 231 27 20 |
France Ipsen Pharma Tél: +33 1 58 33 50 00 | Slovenija Swixx Biopharma d.o.o. Tel: + 386 1 2355 100 |
Hrvatska Swixx Biopharma d.o.o. Tel: +385 1 2078 500 | Slovenská republika Ipsen Pharma, organizacná zložka Tel: + 420 242 481 821 |
Ireland, United Kingdom (Northern Ireland) Ipsen Pharmaceuticals Limited Tel: +44 (0)1753 62 77 77 |
Fecha de la última revisión de este prospecto:
Este medicamento se ha autorizado en «circunstancias excepcionales». Esta modalidad de aprobación significa que debido a la rareza de esta enfermedad no ha sido posible obtener información completa de este medicamento.
La Agencia Europea de Medicamentos revisará anualmente la información nueva de este medicamento que pueda estar disponible y este prospecto se actualizará cuando sea necesario.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
Instrucciones
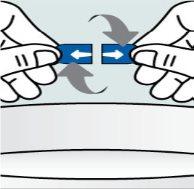
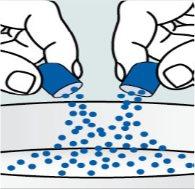
Instrucciones para abrir las cápsulas y espolvorear el contenido sobre algún alimento:
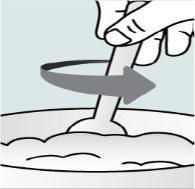
Paso 1. Ponga una pequeña cantidad de un alimento blando en un tazón (2 cucharadas/30 ml de yogur, compota de manzana, puré de plátano o zanahoria, pudín de chocolate, pudín de arroz o copos de avena). El alimento debe estar a temperatura ambiente o más fría.
| Paso 2:
|
| Paso 3:
|
| Paso 4:
|
|
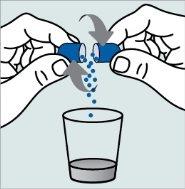
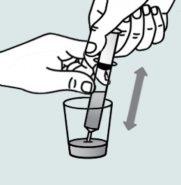
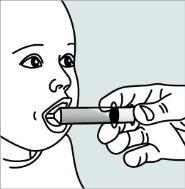
Instrucciones para abrir las cápsulas y espolvorear el contenido en un líquido adecuado para la edad:
No administre el medicamento a través de un biberón o de un vasito para bebés, ya que los gránulos no atravesarán la abertura. Los gránulos no se disolverán en los líquidos.
Póngase en contacto con su farmacia si no tiene una jeringa oral adecuada para administrar el medicamento en casa.
| Paso 1:
|
| |
| Paso 2:
|
Paso 3:
| |
| Paso 4:
|
|
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BYLVAY 200 MICROGRAMOS CAPSULAS DURASForma farmacéutica: CAPSULA, 1200 µgPrincipio activo: odevixibatFabricante: Ipsen PharmaRequiere recetaForma farmacéutica: CAPSULA, 200 - REVISAR µgPrincipio activo: odevixibatFabricante: Ipsen PharmaRequiere recetaForma farmacéutica: CAPSULA, 600 µgPrincipio activo: odevixibatFabricante: Ipsen PharmaRequiere receta
Médicos online para BYLVAY 200 MICROGRAMOS CAPSULAS DURAS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BYLVAY 200 MICROGRAMOS CAPSULAS DURAS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes