
ZOLADEX 3,6 mg IMPLANTE EN JERINGA PRECARGADA

Cómo usar ZOLADEX 3,6 mg IMPLANTE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Zoladex3,6 mg implante en jeringa precargada
goserelina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante parausted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Zoladex 3,6 mg y para qué se utiliza.
- Qué necesita saber antes de empezar a usar Zoladex 3,6 mg.
- Cómo usar Zoladex 3,6 mg.
- Posibles efectos adversos.
- Conservación de Zoladex 3,6 mg.
Contenido del envase e información adicional.
1. Qué es Zoladex 3,6 mg y para qué se utiliza
Zoladex 3,6 mg pertenece a un grupo de medicamentos denominados anti-hormonales, lo que significa que afecta los niveles de diferentes hormonas (sustancias químicas naturales producidas por el organismo). En varones, reducirá los niveles de la hormona masculina, testosterona y, en mujeres, los de la hormona femenina, estrógeno.
Zoladex 3,6 mg se utiliza:
- En varones, para tratar de ciertos tipos de cáncer de próstata.
- En mujeres, para:
- tratar ciertos tipos de cáncer de mama.
- tratar la endometriosis, que es una enfermedad benigna en la que el tejido que normalmente crece dentro del útero, se forma también fuera del útero.
- tratar fibromas uterinos, que son bultos benignos que se forman en el útero.
- disminuir el grosor del revestimiento del útero (endometrio) antes de someterse a una intervención quirúrgica denominada ablación del endometrio, que consiste en la eliminación del revestimiento del útero.
controlar la liberación de óvulos del ovario como parte de un tratamiento para la infertilidad.
2. Qué necesita saber antes de empezar a usar Zoladex 3,6 mg
No use Zoladex 3,6 mg
- Si es alérgico a goserelina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Antes de recibir su inyección, comunique a su médico si está embarazada, piensa estarlo o está en período de lactancia. Zoladex 3,6 mg no debe utilizarse si está embarazada o si está intentando quedarse embarazada, excepto si este medicamento se usa como parte de un tratamiento para la infertilidad. Zoladex 3,6 mg no debe utilizarse durante la lactancia (Ver la sección “Fertilidad, Embarazo y Lactancia” más adelante).
Advertencias y precauciones
- Consulte a su médico o farmacéutico antes de empezar a usar Zoladex 3,6 mg.
- Se han notificado casos de depresión en pacientes que toman Zoladex 3,6 mg que puede ser grave. Si usted está tomando Zoladex 3,6 mg y presenta depresión informe a su médico.
- Informe a su médico si padece alguna afección del corazón o de los vasos sanguíneos o está siendo tratado para ello, incluyendo medicamentos para controlar el ritmo cardíaco (arritmias). El riesgo de problemas del ritmo cardíaco puede aumentar cuando se utiliza Zoladex 3,6 mg.
- Informe a su médico si tiene hipertensión (presión arterial alta).
- Informe inmediatamente a su médico si presenta dolor y hematoma en el abdomen u otros síntomas de hemorragia grave, como dificultad para respirar, mareo, presión arterial baja y/o alteración del nivel de consciencia, que podrían ser el resultado de lesiones vasculares en el lugar de la inyección producidas durante la administración de Zoladex 3,6 mg. (ver sección 4).
- El tratamiento con Zoladex 3,6 mg puede provocar resultados positivos en las pruebas antidopaje.
En caso de ingresar en un hospital comunique al personal sanitario que está siendo tratado con Zoladex 3,6 mg.
Varones:
- Antes de iniciar el tratamiento con este medicamento comunique a su médico si:
- ha padecido alguna dificultad para orinar o ha sufrido molestias en la zona lumbar de la espalda, o
- presenta diabetes.
- Los medicamentos de este tipo pueden causar una pérdida de calcio de los huesos (disminución de su grosor). Si usted presenta alguna enfermedad que afecte a la fortaleza de sus huesos o factores de riesgo para la osteoporosis [por ejemplo abuso crónico de alcohol, ser fumador, tratamiento a largo plazo con anticonvulsivos (medicamentos para la epilepsia o convulsiones) o corticoides (un tipo de medicamentos antiinflamatorios), historial familiar de osteoporosis], comuníquelo a su médico o enfermero.
Mujeres:
- Los medicamentos de este tipo pueden causar una pérdida de calcio de los huesos. Parte de esta pérdida podría recuperarse tras finalizar su tratamiento. Si usted padece alguna enfermedad que afecte la fuerza de sus huesos, o factores de riesgo para la osteoporosis [por ejemplo abuso crónico de alcohol, ser fumadora, tratamiento a largo plazo con anticonvulsivos (medicamentos para la epilepsia o convulsiones) o corticoides, historial familiar de osteoporosis y malnutrición, como la anorexia nerviosa], comuníqueselo a su médico o enfermero, ya que es probable que la reducción de la densidad mineral de sus huesos sea más perjudicial. Si está usando Zoladex 3,6 mg para el tratamiento de endometriosis, su médico podría ponerle un tratamiento adicional para contrarrestar esta reducción del grosor de sus huesos.
- Comunique a su médico si sufre sangrado vaginal que no cesa tras el primer mes de tratamiento con Zoladex 3,6 mg.
- Si está usando Zoladex 3,6 mg para el tratamiento de endometriosis o de fibromas uterinos, la duración máxima de su tratamiento con este medicamento no deberá exceder de un periodo de 6 meses.
- Durante el tratamiento con Zoladex 3,6 mg y hasta que la menstruación se reestablezca una vez interumpido el tratamiento, se deberán emplear métodos anticonceptivos, tales como el preservativo o el diafragma y nunca anticonceptivos orales (“la píldora”). Esta advertencia no aplica cuando se recibe Zoladex 3,6 mg como tratamiento para la infertilidad.
Niños y adolescentes
Zoladex 3,6 mg no está indicado para uso en niños.
Uso de Zoladex 3,6 mg con otros medicamentos
Informe a su médico, farmacéutico o enfermero si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Zoladex 3,6 mg puede interferir con algunos medicamentos utilizados para tratar problemas del ritmo cardíaco (por ejemplo: quinidina, procainamida, amiodarona y sotalol) o puede aumentar el riesgo de problemas del ritmo cardíaco cuando se utiliza con otros medicamentos (por ejemplo: metadona (utilizado para el alivio del dolor y para la desintoxicación de otros medicamentos), moxifloxacino (un antibiótico), antipsicóticos (usados para tratar enfermedades mentales graves)).
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico, farmacéutico o enfermero antes de utilizar este medicamento.
Zoladex 3,6 mg no debe utilizarse si está embarazada o si está intentando quedarse embarazada, excepto si este medicamento se usa como parte de un tratamiento para la infertilidad. Zoladex 3,6 mg no debe utilizarse durante la lactancia.
Conducción y uso de máquinas
No existe evidencia de que Zoladex 3,6 mg altere la capacidad para conducir o utilizar máquinas.
3. Cómo usar Zoladex 3,6 mg
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico.
En caso de duda consulte de nuevo a su médico, farmacéutico o enfermero.
Recuerde que le administren su medicamento.
Su médico le indicará la duración de su tratamiento con Zoladex 3,6 mg. No suspenda el tratamiento antes de que su médico se lo diga.
Zoladex 3,6 mg le será administrado como una inyección por su médico o enfermero, quién seguirá las instrucciones de la etiqueta del envase para una correcta utilización.
Zoladex 3,6 mg es normalmente administrado por inyección bajo la piel, cada 28 días.
Es importante que continúe el tratamiento con Zoladex 3,6 mg incluso si se siente bien, a menos que su médico decida su interrupción.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
- Al administrarle Zoladex 3,6 mg, la inyección podría producirle una lesión en la zona de la administración, incluyendo lesión de vasos sanguíneos del abdomen. En casos muy raros esto ha causado una hemorragia grave. Busque inmediatamente asistencia médica si nota alguno de los siguientes síntomas:
Dolor abdominal, hinchazón del abdomen, dificultad para respirar, mareo, presión arterial baja y/o cualquier alteración del nivel de consciencia. Estos podrían ser síntomas de una hemorragia grave debida a la lesión accidental de algún vaso sanguíneo abdominal producida durante la administración de Zoladex 3,6 mg.
- Efectos adversos comunicados en mujeres:
Muy frecuentes (puede afectar a más de 1 de cada 10 personas)
- Sofocos, sudoración. Estos efectos adversos pueden continuar después de suspender el tratamiento con Zoladex 3,6mg.
- Disminución del deseo sexual.
- Sequedad vaginal.
- Acné, normalmente en el primer mes de tratamiento con Zoladex 3,6 mg.
- Aumento del tamaño de las mamas.
- Reacciones en el lugar de la inyección, como dolor, aparición de moratones, hemorragia, enrojecimiento o hinchazón en la zona, u otras reacciones.
Frecuentes (puede afectar hasta a 1 de cada 10 personas)
- Hormigueo o adormecimiento en los dedos de las manos o de los pies.
- Dolores de cabeza.
- Cambios en la presión arterial (elevación o disminución).
- Erupción cutánea, que suele ser leve y remitir sin interrumpir el tratamiento.
- Caída de cabello (alopecia), que suele ser leve, aunque ocasionalmente puede ser grave y puede también aparecer en mujeres jóvenes.
- Dolor en las articulaciones.
- Aumento de peso.
- Pérdida de la densidad mineral de los huesos (disminución del grosor de los huesos).
- Cambios de humor y depresión (en tratamientos prolongados con Zoladex 3,6 mg).
- Exacerbación y dolor del tumor.
Poco frecuentes (puede afectar hasta a 1 de cada 100 personas)
- Reacciones de hipersensibilidad al medicamento.
- Aumento de los niveles de calcio en su sangre, que puede manifestarse con náuseas, vómitos y/o sed excesivos. Comunique a su médico si nota alguno de estos síntomas, ya que podría tener que realizarle un análisis de sangre.
- Cambios de humor y depresión (en tratamientos cortos con Zoladex 3,6 mg).
Raros (puede afectar hasta a 1 de cada 1.000 personas)
- Reacción anafiláctica (reacción alérgica grave).
- Quistes en el ovario.
Sobreestimulación de los ovarios cuando Zoladex 3,6 mg se utiliza como parte de un tratamiento para la infertilidad. Si nota dolor abdominal, hinchazón abdominal, nauseas o vómitos después del tratamiento con estos medicamentos, comuníqueselo inmediatamente a su médico.
Muy raros (puede afectar hasta a 1 de cada 10.000 personas)
- Desarrollo de un tumor en la hipófisis (glándula endocrina que está en la cabeza). Si tiene un tumor en la hipófisis, este medicamento puede provocar sangrado del tumor. Los tumores en la hipófisis pueden causar dolor de cabeza, malestar, pérdida de visión e incluso pueden hacer que pierda la consciencia.
- Trastornos psicóticos que pueden hacer que tenga alucinaciones, trastornos del pensamiento y cambios de personalidad.
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles)
- Hemorragia vaginal al inicio del tratamiento.
- Inflamación de la vagina.
- flujo vaginal.
- Si tiene cáncer de mama puede notar un empeoramiento de su enfermedad, como un aumento del dolor y/o aumento del tamaño del tejido afectado. Estos síntomas aparecen al principio y desaparecen al continuar el tratamiento.
- Menopausia. Al finalizar el tratamiento con Zoladex 3,6 mg podría no volver a tener la regla. Se desconoce si esto es debido al efecto de Zoladex.
- Si tiene fibromas en el útero, puede notar un ligero aumento de los síntomas, como por ejemplo del dolor. Si los síntomas persisten o se siente incómoda, comuníqueselo a su médico.
- Alteraciones en el electrocardiograma (prolongación del intervalo QT).
- Cambios en el número de las células de la sangre (observado en un análisis de sangre).
- coágulos de sangre en los pulmones (que causan dolor en el pecho y dificultad para respirar) e inflamación del tejido que rodea las estructuras (alveolos) de los pulmones, donde se absorbe el oxígeno (neumonía intersticial) (que causa síntomas como tos y dificultad para respirar).
- Alteración del hígado.
- Niveles altos de calcio en la sangre, que puede aparecer al inicio del tratamiento en pacientes con cáncer de mama y metástasis.
- Nerviosismo, alteración del sueño.
- Retención de líquidos en las extremidades (edema periférico).
- Alteraciones de la voz.
- Cambios en el vello corporal.
- Nauseas, vómitos, diarrea, estreñimiento, dolor abdominal.
- Aumento del colesterol en sangre.
- Piel seca.
- Dolor muscular.
- Calambres en las pantorrillas.
- Cansancio.
- Efectos adversos comunicados en varones:
Muy frecuentes (puede afectar a más de 1 de cada 10 personas)
- Sofocos, sudoración. Estos efectos adversos pueden continuar después de suspender el tratamiento con Zoladex 3,6mg.
- Disminución del deseo sexual e impotencia.
Frecuentes (puede afectar hasta a 1 de cada 10 personas)
- Aumento del nivel de azúcar en su sangre.
- Cambios de humor y depresión (en tratamientos prolongados).
- Hormigueo o adormecimiento en los dedos de las manos o de los pies (parestesia).
- Compresión de la médula espinal.
- Erupción cutánea, que generalmente es leve y a menudo remite sin interrumpir el tratamiento.
- Función cardiaca disminuida, infarto. El riesgo de desarrollarlos es mayor cuando se utiliza Zoladex junto con otros medicamentos (antiandrógenos) para tratar el cáncer de próstata.
- Cambios en la presión arterial (elevación o disminución).
- Dolor de huesos, generalmente al inicio del tratamiento con Zoladex 3,6 mg. Si le ocurre esto comuníqueselo a su médico, ya que podría tener que recetarle un medicamento para aliviar el dolor.
- Aumento de peso.
- Hinchazón de las mamas.
- Reacciones en el lugar de la inyección, como dolor, aparición de moratones, hemorragia, enrojecimiento o hinchazón en la zona, u otras reacciones.
- Pérdida de la densidad mineral de los huesos (disminución del grosor de los huesos).
Poco frecuentes (puede afectar hasta a 1 de cada 100 personas)
- Reacciones de hipersensibilidad al medicamento.
- Dolor en las articulaciones.
- Molestias en las mamas.
- Cambios de humor y depresión (en tratamientos cortos).
- Obstrucción de los uréteres (conductos que transportan la orina desde los riñones hasta la vejiga), que puede causar dificultad para orinar o molestias en la zona lumbar de la espalda.
Raros (puede afectar hasta a 1 de cada 1.000 personas)
- Reacción anafiláctica (reacción alérgica grave).
Muy raros (puede afectar hasta a 1 de cada 10.000 personas)
- Trastornos psicóticos que pueden hacer que tenga alucinaciones, trastornos del pensamiento y cambios de personalidad.
- Desarrollo de un tumor en la hipófisis (una glándula endocrina que está en la cabeza). Si tiene un tumor en la hipófisis, Zoladex 3,6 mg puede provocar sangrado del tumor. Los tumores en la hipófisis pueden causar dolor de cabeza, malestar, pérdida de visión e incluso pueden hacer que pierda la consciencia.
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles)
- Caída de cabello, especialmente pérdida de vello corporal.
- Alteraciones en el electrocardiograma (prolongación del intervalo QT).
- Cambios en el número de las células de la sangre (observado en un análisis de sangre).
- Coágulos de sangre en los pulmones (que causan dolor en el pecho y dificultad para respirar) e inflamación del tejido que rodea las estructuras (alveolos) de los pulmones donde se absorbe el oxígeno (neumonía intersticial) (que causa síntomas como tos y dificultad para respirar).
- Alteración del hígado.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico, o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Zoladex 3,6 mg
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 25ºC.
Conservar en el embalaje original.
No utilice Zoladex 3,6 mg después de la fecha de caducidad que aparece en el envase y el sobre después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Zoladex 3,6 mg
- El principio activo es goserelina (como acetato). Cada implante contiene 3,6 mg de goserelina.
- El otro componente es copolímero láctido-glicólido.
Aspecto del producto y contenido del envase
El medicamento se presenta en forma de implante de 3,6 mg en una jeringa precargada dentro de un sobre sellado.
El implante es estéril, color crema y libera el fármaco de forma prolongada.
El sobre contiene en su interior, además, un desecante.
La jeringa precargada dispone de un dispositivo de seguridad (clip-rojo) y de un sistema de protección de la aguja.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
AstraZeneca Farmacéutica Spain, S.A.
C/ Puerto de Somport 21-23
28050 Madrid
España
Responsable de la fabricación
AstraZeneca AB
Gärtunavägen
SE-152 57 Södertälje
Suecia
Otras presentaciones
Zoladex Trimestral 10,8 mg: Envase conteniendo un implante de 10,8 mg en una jeringa precargada dentro de un sobre sellado, que además contiene en su interior un desecante. La jeringa precargada dispone de un dispositivo de seguridad (clip-azul) y de un sistema de protección de la aguja.
Fecha de la última revisión de esteprospecto: Febrero 2020
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
<----------------------------------------------------------------------------------------------------------------------------------?
Esta información está destinada únicamente a profesionales sanitarios:
Zoladex 3,6 mg se debe administrar mediante inyección subcutánea – lea y comprenda todas las instrucciones completamente antes de su administración.
- Tumbar al paciente en una posición cómoda, con la parte superior del cuerpo ligeramente elevada. Limpiar la zona abdominal de la inyección con un algodón impregnado en un agente desinfectante (alcohol, etc.).
NOTA: Se debe tener precaución mientras se procede a la inyección de Zoladex 3,6 mg en la pared abdominal anterior, debido a la proximidad de la arteria epigástrica inferior subyacente y a sus ramificaciones. Los pacientes muy delgados podrían tener un alto riesgo de lesión vascular.
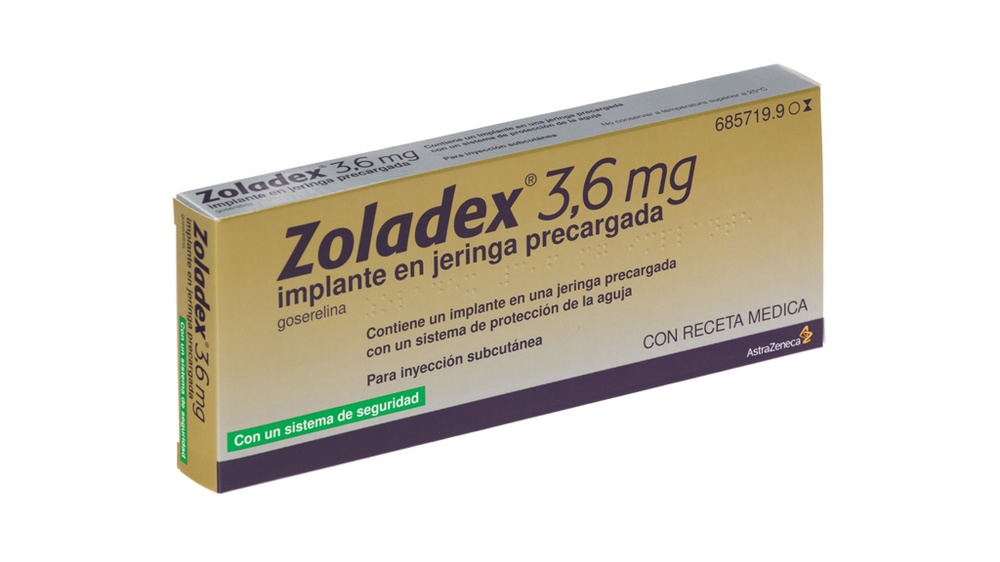
- Examinar el sobre y la jeringa por si estuvieran dañados. Retirar la jeringa del sobre abierto y mantenerla inclinada hacia la luz.
Comprobar que se observa al menos parte del implante de Zoladex 3,6 mg. (Figura 1).
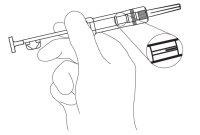 Figura 1
Figura 1
- Retirar suavemente la pestaña de plástico de seguridad de la jeringa de color rojo y desecharla. (Figura 2).
Retirar el capuchón que protege la aguja. Al no ser un inyectable líquido, no hay necesidad de eliminar las burbujas de aire, ya que al intentarlo el implante de Zoladex 3,6 mg podría desplazarse.
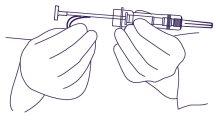 Figura 2
Figura 2
- Sujetar la jeringa alrededor del sistema de protección, utilizando una técnica aséptica. Coger un pliegue de la piel del paciente e introducir la aguja con un ángulo de inclinación poco pronunciado (30 a 45 grados).
Con la apertura de la aguja hacia arriba, introducir la aguja en el tejido subcutáneode la pared abdominal anterior debajo de la línea del ombligo, hasta que el sistema de protección toque la piel del paciente. (Figura 3).
 Figura 3
Figura 3
NOTA: La jeringa de Zoladex 3,6 mg no puede utilizarse para la aspiración. Si la aguja hipodérmica penetra en un vaso grande, la sangre se verá al instante en la cámara de la jeringa. Si se penetra un vaso, retirar la aguja y controlar inmediatamente cualquier sangrado resultante, monitorizando al paciente para cualquier signo o síntoma de hemorragia abdominal. Después de asegurarse que el paciente está hemodinámicamente estable, se puede inyectar otro implante de Zoladex 3,6 mg con una nueva jeringa en otra zona. Extremar la precaución cuando administre Zoladex 3,6 mg en los pacientes con un IMC bajo y/o para los pacientes que reciben dosis anticoagulantes completas.
- No penetrar el músculo, ni el peritoneo.En la Figura 4, a continuación, se muestran una sujeción y ángulo de exposición incorrectos.
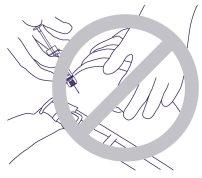 Figura 4
Figura 4
- Presionar el émbolo completamente,hasta que no pueda presionar más, con el fin de depositar el implante de Zoladex 3,6 mg y de activar el sistema de protección. Podría oír un “click” y notar cómo se activa el sistema de protección, deslizándose automáticamente para recubrir la aguja. Si el émbolo no se presiona completamente, el sistema de protección NOse activará.
NOTA:La aguja no se retrae.
- Continuar sujetando la jeringa como se muestra en la Figura 5, retirar la aguja permitiendo
que el sistema de protección continúe deslizándose y cubriendo la aguja.
Desechar la jeringa en un contenedor, de acuerdo con la normativa local.
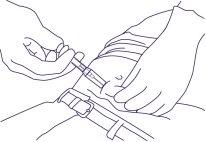 Figura 5
Figura 5
NOTA: En el improbable caso de la necesidad de eliminar quirúrgicamente un implante de Zoladex 3,6 mg, éste puede ser localizado por ultrasonido.
- País de registro
- Precio medio en farmacia106.31 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ZOLADEX 3,6 mg IMPLANTE EN JERINGA PRECARGADAForma farmacéutica: IMPLANTE, 10,8 mgPrincipio activo: goserelinaFabricante: Astrazeneca Farmaceutica Spain S.A.Requiere recetaForma farmacéutica: INYECTABLE, 42 mgPrincipio activo: leuprorelinFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: INYECTABLE, 0,1 mgPrincipio activo: triptorelinaFabricante: Ipsen Pharma S.A.Requiere receta
Médicos online para ZOLADEX 3,6 mg IMPLANTE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ZOLADEX 3,6 mg IMPLANTE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes












