
XEMBIFY 200 mg/ml SUBCUTANEOUS INJECTABLE SOLUTION

How to use XEMBIFY 200 mg/ml SUBCUTANEOUS INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Xembify 200mg/ml solution for subcutaneous injection
Human normal immunoglobulin (IgSC)
This medicinal product is subject to additional monitoring, which will allow for the quick detection of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
? Keep this leaflet, you may need to read it again.
? If you have any further questions, ask your doctor, pharmacist, or nurse.
? This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
? If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Xembify and what is it used for
- What you need to know before you use Xembify
- How to use Xembify
- Possible side effects
- Storage of Xembify
- Contents of the pack and other information
1. What is Xembify and what is it used for
What is Xembify
Xembify is a solution of human immunoglobulins (antibodies, mainly immunoglobulin G) to help your body fight infections.
Xembify contains immunoglobulins from the plasma of healthy individuals. Immunoglobulins help fight infections caused by bacteria and viruses. The medicine works exactly like the immunoglobulins naturally present in human blood produced by the immune system.
What is Xembify used for
You are using Xembify because you have very low levels of immunoglobulins due to a medical condition called immunodeficiency. Xembify infusions increase the levels of immunoglobulin (antibodies), specifically immunoglobulin G (IgG), in your blood to normal levels.
This medicine is for adults, children, and adolescents (0-18 years) who lack sufficient antibodies (replacement therapy):
- Patient with primary immunodeficiency syndrome (PIDS) with a congenital antibody deficiency.
- Hypogammaglobulinemia (a disease that involves low levels of immunoglobulins in the blood) and recurrent bacterial infections in patients with chronic lymphocytic leukemia (a blood cancer where too many white blood cells are produced) in whom prophylactic antibiotics have not been effective.
- Hypogammaglobulinemia and recurrent bacterial infections in patients with multiple myeloma (a tumor formed by cells derived from bone marrow).
Hypogammaglobulinemia in patients after a stem cell transplant (allogeneic hematopoietic stem cell transplantation, HSCT), when receiving stem cells from another person.
2. What you need to know before you use Xembify
Do not use Xembify
? if you are allergic to human normal immunoglobulin or to any of the other ingredients of this medicine (listed in section 6);
? if you have had a severe allergic reaction (such as anaphylaxis) to human immunoglobulin;
? if you have antibodies against immunoglobulin A (IgA) in your blood. This may occur if you have an IgA deficiency. Since Xembify contains IgA, you may have an allergic reaction;
? by injection into a blood vessel (intravenously) or into a muscle (intramuscularly).
Tell your doctor, pharmacist, or nurse before Xembify infusion if you have ever had any side effects from an immunoglobulin or any of its components.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting Xembify.
- Tell your doctor if you have a history of heart disease, vascular disease, a blood clot in a blood vessel (such as a stroke, heart attack, or pulmonary embolism), thick blood, diabetes mellitus, high blood pressure, a bleeding or clotting disorder, or if you have been immobile for some time. Tell your doctor if you are taking estrogens, usually as contraceptives. You may have a higher risk of developing a blood clot after Xembify infusion. Contact your doctor immediately if you experience difficulty breathing; chest pain; pain and swelling of an arm or leg; or weakness or numbness on one side of the body. You may have a blood clot in a blood vessel.
- Contact your doctor if you experience severe headache, stiff neck, drowsiness, fever, extreme sensitivity to light, nausea, or vomiting. These side effects may occur hours or even days after Xembify infusion. You may have aseptic meningitis syndrome.
- Xembify may cause kidney problems, including kidney failure. Tell your doctor if you have reduced kidney function.
- Xembify may interfere with certain blood tests (serological tests). Always tell your doctor that you are being treated with Xembify before having a blood test.
Allergic reactions
Allergic reactions are rare. However, you may be allergic to immunoglobulins without knowing it. Allergic reactions such as a sudden drop in blood pressure or anaphylactic shock (a sudden drop in blood pressure with other symptoms such as throat swelling, difficulty breathing, and skin rash) are rare but may occur occasionally, even if you have not had side effects from immunoglobulins in the past. You have a higher risk of allergic reactions if you have an IgA deficiency with anti-IgA antibodies. Make sure to tell your doctor if you have an IgA deficiency. Xembify contains some amount of IgA, which may increase the risk of an allergic reaction. See section 4 of this leaflet (Possible side effects) for signs and symptoms of an allergic reaction.
Risk of transmission of diseases
Xembify is purified from human plasma obtained from healthy donors. When biological medicines are administered, the possibility of transmitting infectious diseases due to the transmission of pathogens cannot be entirely excluded. However, in the case of products prepared from human plasma, the risk of transmitting pathogens is reduced by: (1) epidemiological controls of the donor population and selection of individual donors through a medical interview; (2) analysis of individual donations and plasma pools for detection of viral infection markers; and (3) manufacturing procedures with demonstrated capability to inactivate/eliminate pathogens.
Despite these measures, when medicines prepared from human blood or plasma are administered, the possibility of transmitting infections cannot be entirely excluded. This also applies to unknown or emerging viruses or other types of infections.
The measures taken are considered effective against enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus, and against non-enveloped viruses such as hepatitis A virus. The measures taken may have limited value against non-enveloped viruses such as parvovirus B19.
Immunoglobulins have not been associated with hepatitis A or parvovirus B19 infections, possibly because the antibodies against these infections, contained in the medicine, are protective.
It is strongly recommended that each time you are given a dose of this medicine, the name and batch number of the medicine (which appears on the label and carton after Batch) be recorded in order to maintain a record of the batches used.
Children and adolescents
The warnings and precautions apply to adults, children, and adolescents.
Other medicines and Xembify
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Always administer Xembify infusion alone, without mixing it with any other medicine.
If you are going to be vaccinated, tell your doctor that you are being treated with Xembify. Xembify may interfere with some vaccines (live virus vaccines) such as measles, mumps, rubella, and varicella. You may need to wait up to 3 months after receiving Xembify before being vaccinated. For measles vaccine, you may need to wait up to 1 year.
These interactions apply to children, adults, and the elderly.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
No studies have been performed with Xembify in pregnant or breastfeeding women, and your doctor or pharmacist will guide you. Clinical experience with immunoglobulins suggests that no harmful effects on pregnancy, the fetus, or the baby are expected. If you are breastfeeding, the immunoglobulins contained in Xembify may also be found in breast milk. Therefore, they may protect your baby from certain infections. Clinical experience with immunoglobulins suggests that no harmful effects on fertility are expected.
Driving and using machines
Your ability to drive and use machines may be affected by some side effects, such as dizziness, associated with Xembify. If you experience side effects during treatment, wait until they disappear before driving or using machines.
3. How to use Xembify
Follow your doctor's administration instructions for this medication exactly. If in doubt, consult your doctor again.
Xembify should be infused under the skin (subcutaneous or SC administration).
Treatment with Xembify will be started by your doctor or nurse. Do not start treatment at home with Xembify until you have received complete instructions.
Dose
Your doctor will determine the recommended dose and administration schedule. Your doctor will calculate the correct dose for you based on your body weight, any previous treatment you have received, and your response to treatment. Follow your doctor's, pharmacist's, or nurse's administration instructions exactly as contained in this leaflet or as indicated. If in doubt, ask them.
Your first dose may be what is called a "loading dose", which aims to rapidly increase the levels of immunoglobulin in your blood. Your doctor will determine if you need a "loading dose" (for adults or children) of at least 1 to 2.5 ml/kg of body weight. You may receive this loading dose divided over several days.
You will be given Xembify periodically, from daily to once every 2 weeks; the monthly cumulative dose will be approximately 1.5 to 5 ml/kg of body weight. Your doctor may adjust the dose based on your response to treatment.
Do not change this dose or the time interval until the next dose without consulting your doctor first.
If you think you should receive a different dose or want to change your administration schedule, consult your doctor first. Contact your doctor if you miss a dose.
Routine blood tests will be performed to measure the level of immunoglobulin in your blood. Discuss scheduling with your doctor.
There is no difference between the dose for adults, including elderly people (65 years or older), and the dose for children and babies, as the amount of Xembify infused is based on body weight.
Method of administration
Subcutaneous infusion for home treatment must be started and supervised by a healthcare professional with experience in guiding patients for home treatment. Suitable infusion pumps for subcutaneous administration of immunoglobulins can be used. The patient or caregiver must receive training on the use of an infusion pump, infusion techniques, maintaining a treatment diary, and recognizing and taking measures in case of severe adverse reactions.
You (or your caregiver) must receive training on:
- the use of an administration device, such as a syringe pump, if necessary,
- the use of aseptic infusion techniques (without germs),
- maintaining a treatment diary, and
- recognizing and taking measures in case of severe adverse reactions.
Follow your doctor's instructions carefully regarding the dose, infusion rate, and infusion schedule of Xembify for the treatment to be effective.
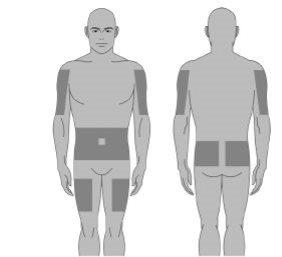
Infusion sites
Administer Xembify only by subcutaneous route. Infuse Xembify into the subcutaneous tissue at sites such as
- the abdomen,
- the thighs,
- the upper arms, and
- the lateral hip.
When selecting infusion sites, avoid: bony areas, visible blood vessels, scars, and any area of inflammation (irritation) or infection. Alternate sites for each administration.
In your initial two infusions, you will start with an infusion rate of 10 ml per hour per infusion site. If you do not experience adverse reactions (see section 4), the rate can be increased every 10 minutes to a maximum of 20 ml per hour per infusion site in children and adolescents and 25 ml per hour per infusion site in adults. After 2 infusion sessions, the administration rate can be gradually increased up to 35 ml per hour per infusion site. Consult your doctor before increasing the infusion rate.
You can perform the infusion simultaneously at more than one site, provided they are at least 5 cm apart. Adults can divide the dose between several sites, especially if the volume is more than 30 ml. There is no limit to the number of sites you can use. You can use more than one pump to do so.
Instructions for use
Subcutaneous infusion for home treatment must be started and supervised by a healthcare professional with experience in guiding patients for home treatment. Suitable infusion pumps for subcutaneous administration of immunoglobulins can be used. The patient or caregiver must receive training on the use of an infusion pump, infusion techniques, maintaining a treatment diary, and recognizing and taking measures in case of severe adverse reactions.
Follow these steps and use an aseptic technique to administer Xembify.
Before use, let the solution reach room or body temperature (20-37°C). This may take 60 minutes or more.
Do not apply heat or put it in the microwave.
Step1: Preparation of materials
Gather the Xembify vial(s), ancillary materials, sharps container, patient treatment diary/log, and infusion pump(s).
Step2: Surface cleaning
Set up your infusion area on a clean, flat, non-porous surface, such as a kitchen counter.
Avoid using porous surfaces like wood. Clean the surface with an alcohol wipe in a circular motion from the center out.
Step3: Hand washing
Wash and dry your hands well before using Xembify.
Your doctor may recommend using antibacterial soap or wearing gloves.
Step4: Vial inspection
The liquid in the vial should be clear to slightly opalescent, colorless, or pale yellow or light brown.
Do not use the vial if:
- the solution is cloudy or discolored. The solution should be clear to slightly opalescent, colorless, or pale yellow or light brown.
- the protective cap is missing or there is evidence of tampering. Report immediately to your doctor.
- the expiration date has passed.
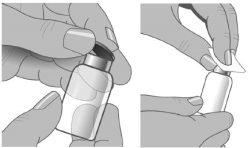
Step5: Removal of the protective cap
Remove the protective cap from the vial to expose the center of the stopper.
Clean the stopper with alcohol and let it dry.
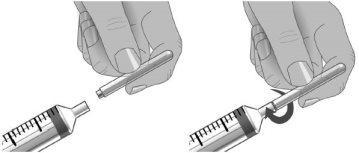
Step6: Transfer of Xembify from the vial(s) to the syringe
Do not let your fingers or other objects touch the inner plunger rod, the tip of the needle, or other areas that may come into contact with the Xembify solution. Ensure that the needles have the cap on until use and that the needles and syringes remain in the clean area created in step 2. This is called "aseptic technique" to avoid germs entering Xembify.
Using aseptic technique, attach each needle to the tip of the syringe.
Step7: Preparation of the syringe and withdrawal of the Xembify solution into the syringe
Remove the needle cap.
Pull the plunger rod of the syringe to the level that corresponds to the amount of Xembify to be withdrawn from the vial.
Place the Xembify vial on a flat, clean surface and insert the needle into the center of the vial stopper.
Inject air into the vial. The amount of air should match the amount of Xembify to be withdrawn.
Invert the vial and withdraw the correct amount of Xembify. If multiple vials are needed to obtain the correct dose, repeat steps 4-7.
Administer immediately after transferring Xembify from the vial to a syringe.
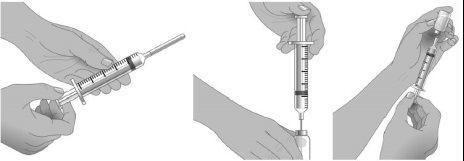
Step8: Preparation of the infusion pump
Follow the manufacturer's instructions to prepare the infusion pump, administration tubing, and Y-connector, if necessary.
Prime the administration tubing with Xembify to remove any air from the tubing or needle. To prime, hold the syringe in one hand and the needle with the administration tubing cap in the other. Gently press on the plunger until you see a drop of Xembify come out of the needle.
Step9: Selection of the number and location of infusion sites
Select one or more infusion sites according to your doctor's instructions.
The number and location of infusion sites depend on the total dose volume.
Suitable sites for infusion are: the abdomen, thighs, upper arms, and lateral hip.
Avoid: bony areas, visible blood vessels, scars, and any area of inflammation (irritation) or infection.
Alternate sites between future infusions.
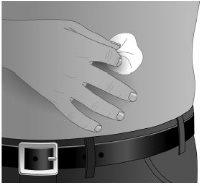
Step10: Preparation of the infusion site
Clean the infusion site(s) with a sterile alcohol wipe, starting from the center of each site and moving out in a circular motion. Let the site(s) dry (at least 30 seconds).
Before infusion, the sites must be clean, dry, and at least 5 cm apart.
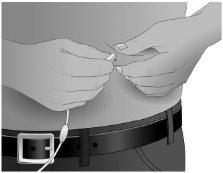
Step11: Insertion of the needle
Hold the skin between two fingers (pinch at least 2.5 cm of skin) and insert the needle at a 90-degree angle into the tissue under the skin or subcutaneous tissue.
Step12: Checking that the needle is not in a blood vessel
After inserting each needle into the tissue (and before infusion), ensure that it has not accidentally entered a blood vessel. To do this, attach a sterile syringe to the end of the primed administration tubing. Pull back on the syringe plunger and observe if blood enters the administration tubing.
If you see blood, remove and discard the needle and administration tubing.
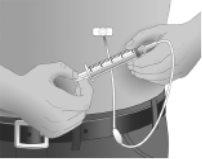
Repeat the priming and needle insertion steps using a new needle and administration tubing, and a new infusion site.
Secure the needle in place by putting a sterile gauze or transparent dressing over the site.
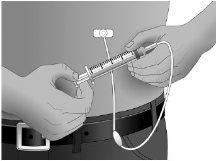
Step13: Repeating for other sites, as necessary
Step14: Infusion of Xembify
Infuse Xembify as soon as possible after preparation.
Follow the manufacturer's instructions to fill the tubing and use the infusion pump.
Step15: After infusion
Follow the manufacturer's instructions to turn off the pump.
Remove and discard any dressing or adhesive tape.
Carefully remove the inserted needle(s) or catheter(s).
Discard any unused solution in an appropriate waste container, according to instructions.
Discard any used administration equipment in an appropriate waste container.
Store your materials in a safe place.
Follow the manufacturer's instructions for the care of the syringe pump.
Step16: Recording each infusion
Remove the detachable label with the product batch number from the Xembify vial and use it to complete the patient log. Include information about each infusion, such as:
- the time and date,
- the dose,
- the batch number(s),
- the infusion sites, and
- any reaction.
Remember to bring your diary when you visit your doctor. Your doctor may ask you to show them your treatment diary/log.
Inform your doctor about any problems you have during your infusions. Call your doctor to report adverse effects.
Use in elderly patients
The dose in elderly patients is not considered different from that in patients aged 18 to 65 years.
Use in children and adolescents
The dose in children and adolescents (0-18 years) is not different from that in adults. In infants and children, the infusion site may be changed every 5 to 15 ml.
If you use more Xembify than you should
Contact your doctor for instructions.
In case of overdose or accidental administration, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount administered.
If you miss a dose of Xembify
Do not take a double dose to make up for missed doses. Contact your doctor for instructions.
If you have any other questions about the use of this medication, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Rarely, human normal immunoglobulins can cause a sudden drop in blood pressure and, in isolated cases, anaphylactic shock, even when the patient has not shown hypersensitivity to previous administration.
The signs or symptoms of these rare allergic reactions include:
- feeling of dizziness, dizziness, or fainting;
- skin rash and itching, swelling of the mouth or throat, difficulty breathing, wheezing;
- irregular heartbeat, chest pain, bluish discoloration of the lips or fingers and toes.
If you observe any signs of an allergic reaction or anaphylactic-type reaction during the infusion of Xembify, interrupt the infusion immediately and contact your doctor or go to the nearest hospital. See section 2 of this leaflet (Warnings and precautions). If you observe any of these signs during the infusion of Xembify when administered by a healthcare professional, inform your doctor or nurse immediately. They will decide whether to reduce the infusion rate or stop it altogether.
Local reactions may occur at the infusion sites, such as swelling, pain, redness, induration (hard lump), local heat, itching, bruising, and skin rash.
Xembify may occasionally cause chills, headache, dizziness, fever, vomiting, allergic reactions, general discomfort (nausea), joint pain, low blood pressure, and moderate lower back pain.
The following side effects are very common with Xembify (may affect 1 or more people in every10):
? Local reaction at the infusion site
The following side effects are common with Xembify (may affect 1 or more people in every100):
? Headache
? Arthralgia (joint pain)
? Back pain
? Rhinitis (nasal discharge, sneezing, and congestion)
? Diarrhea
? Nausea
? Pyrexia (fever)
? Decreased immunoglobulin G in blood
? Pruritus (itching)
? Papule (small elevated area of the skin)
Post-marketing side effects
The following adverse reactions have been identified and reported during post-marketing use of Xembify (none serious): dyspnea (difficulty breathing), fatigue, pain, nausea, headache, and local reaction at the infusion site, such as erythema (redness) and swelling. It is not always possible to reliably estimate the frequency of these reactions.
Reporting side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System: www.notificaRAM.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Xembify
Keep this medicine out of the sight and reach of children.
- Store in a refrigerator (between 2°C and 8°C).
Xembify can be stored at temperatures not exceeding 25°C for a maximum of 6 months at any time before the expiry date.
On the day the medicine is removed from the refrigerator, write the date 6 months later or the expiry date printed on the carton flap, whichever is earlier, in the "Disposal Date" space provided on the carton.
If stored at room temperature, do not put the medicine back in the refrigerator. Use the medicine before the "Disposal Date" or discard it.
- Do not freeze.
- Keep the vial in the outer packaging to protect it from light.
- Administer as soon as possible after transferring Xembify from the vial to the syringe.
Do not use this medicine after the expiry date stated on the label and carton.
Do not use this medicine if you notice it is discolored, cloudy, has sediment, or has been frozen.
Medicines should not be disposed of via wastewater or household waste. Dispose of containers and unused medicines at the pharmacy's SIGRE Point. If in doubt, ask your pharmacist how to dispose of containers and unused medicines. This will help protect the environment.
6. Package Contents and Additional Information
Xembify Composition
The active substance is normal human immunoglobulin (IgSC). One milliliter contains 200 mg of normal human immunoglobulin, of which at least 98% is IgG.
The percentage of IgG subclasses is approximately 62% IgG1, 30% IgG2, 4.3% IgG3, and 3.2% IgG4. It contains some amount of IgA (not more than 160 micrograms/ml).
The other ingredients are glycine (E 640), polysorbate 80 (E 433), and water for injectable preparations.
Product Appearance and Package Contents
Xembify is a subcutaneous injectable solution. The solution is transparent to slightly opalescent, colorless, or pale yellow or light brown.
Xembify is presented in a carton and is contained in a transparent glass vial with a stopper, an aluminum cap, a plastic cap, and a security seal that ensure the integrity of the packaging.
Xembify is available in package sizes of
1 or 10 vials containing 1 g of normal human immunoglobulin in 5 ml of subcutaneous injectable solution
1, 10, or 20 vials containing 2 g of normal human immunoglobulin in 10 ml of subcutaneous injectable solution
1 or 20 vials containing 4 g of normal human immunoglobulin in 20 ml of subcutaneous injectable solution
1 or 10 vials containing 10 g of normal human immunoglobulin in 50 ml of subcutaneous injectable solution
Each carton contains 1, 10, or 20 vials of Xembify and 1 leaflet.
Not all package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Instituto Grifols, S.A.
Can Guasc, 2 - Parets del Vallès
08150 Barcelona - Spain
Date of Last Revision of this Leaflet: May 2024
Other Sources of Information
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to XEMBIFY 200 mg/ml SUBCUTANEOUS INJECTABLE SOLUTIONDosage form: INJECTABLE, 165 mg/mlActive substance: immunoglobulins, normal human, for extravascular adm.Manufacturer: Octapharma S.A.Prescription requiredDosage form: INJECTABLE, 200 mg/mlActive substance: immunoglobulins, normal human, for extravascular adm.Manufacturer: Baxalta Innovations GmbhPrescription requiredDosage form: INJECTABLE, 200 mg/mlActive substance: immunoglobulins, normal human, for extravascular adm.Manufacturer: Csl Behring GmbhPrescription required
Online doctors for XEMBIFY 200 mg/ml SUBCUTANEOUS INJECTABLE SOLUTION
Discuss questions about XEMBIFY 200 mg/ml SUBCUTANEOUS INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















