WAYLIVRA 285 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE

How to use WAYLIVRA 285 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Waylivra 285 mg solution for injection in a pre-filled syringe
volanesorsen
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Waylivra and what is it used for
- What you need to know before you use Waylivra
- How to use Waylivra
- Possible side effects
- Storage of Waylivra
- Contents of the pack and other information
1. What is Waylivra and what is it used for
Waylivra contains the active substance volanesorsen, which helps to treat a disease called familial chylomicronemia syndrome (FCS). FCS is a genetic disease that leads to abnormally high levels of a type of fat called triglycerides in the blood. This can lead to inflammation of the pancreas, which is very painful. Along with a low-fat diet, Waylivra helps to reduce the concentration of triglycerides in the blood.
You may be prescribed Waylivra after you have received other medicines to reduce triglyceride levels in the blood that have not been effective enough.
You will only receive Waylivra if a genetic test confirms that you have FCS and it is considered that your risk of pancreatitis is very high.
During treatment with Waylivra, you must continue with the low-fat diet that your doctor has prescribed for you.
This medicine is for patients aged 18 and over.
2. What you need to know before you use Waylivra
Do not use Waylivra:
- if you are allergic to volanesorsen or any of the other ingredients of this medicine (listed in section 6).
- if you have a disorder called thrombocytopenia, which means you have very few platelets in your blood (less than 140 x 10^9/l). You may notice this if you have a wound that bleeds and takes a long time to stop (more than 5 to 6 minutes for a scratch on the skin). Your doctor will do a blood test to check this before giving you this medicine. You may not know you have this condition or what may have caused it.
If any of the above applies to you, or if you are not sure, consult your doctor, nurse, or pharmacist before using Waylivra.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to use Waylivra if you have or have had any of the following medical problems:
- Very high triglyceride levels, but not due to FCS.
- A low number of platelets (thrombocytopenia), a type of blood cell that helps blood to clot; before you start using this medicine, your doctor will do a blood test to measure the number of platelets you have in your blood.
- Liver or kidney problems of any kind.
Blood tests
Before using this medicine, your doctor will do a test to measure the number of platelets and then at regular intervals while you are receiving Waylivra to check the number of platelets.
You should consult your doctor immediately if you have any signs that indicate a low platelet count, such as unusual or prolonged bleeding, red spots on the skin (called petechiae), unexplained bruising, bleeding that does not stop, nosebleeds, or if you have stiffness in the neck or a severe headache.
Your doctor may also ask you to have a blood test every 3 months to check for any liver problems. You should consult your doctor immediately if you have signs of liver damage, such as yellowing of the skin and eyes, pain or swelling of the abdomen, dizziness, confusion, or a general feeling of being unwell.
If necessary, your doctor may change the frequency of administration of this medicine or stop it for a while. You may need to consult a specialist doctor in blood disorders to determine if you can continue to receive Waylivra.
Urine tests
Your doctor may ask you to have urine or blood tests every 3 months to check the condition of your kidneys. You should consult your doctor immediately if you have signs of kidney damage, such as swelling in your ankles, legs, and feet, decreased amount of urine, difficulty breathing, dizziness, confusion, or intense fatigue or drowsiness.
Diet
Before starting to use this medicine, you should be on a diet designed to help lower your triglyceride levels in the blood.
It is important that you continue with this diet to lower triglycerides while you are receiving Waylivra.
Children and adolescents
Do not use Waylivra if you are under 18 years old. Waylivra has not been studied in patients under 18 years old.
Other medicines and Waylivra
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. It is important that you inform your doctor if you are receiving treatment with any of the following:
- Medicines to prevent blood clots from forming, e.g., acetylsalicylic acid, dipyridamole, or warfarin.
- Other medicines that may affect blood clotting, such as non-steroidal anti-inflammatory drugs like ibuprofen, medicines used to prevent heart attacks and strokes, such as clopidogrel, ticagrelor, and prasugrel, antibiotics like penicillin, medicines like ranitidine (used to reduce stomach acid) and quinine (used to treat malaria).
- Medicines that may cause liver problems, such as paracetamol.
Using Waylivra with alcohol
The effect of using Waylivra with alcohol is not known. You should avoid drinking alcohol during treatment with this medicine, due to the risk of liver problems.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. It is recommended to avoid using Waylivra during pregnancy.
Driving and using machines
Waylivra is unlikely to affect your ability to drive or use machines.
Waylivra contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose, which is essentially "sodium-free".
3. How to use Waylivra
Follow the instructions for administration of this medicine exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist again.
Before administering this medicine, your doctor should rule out other causes of elevated triglycerides, such as diabetes or thyroid problems.
Your doctor will tell you how often you should use this medicine. They may change how often you use it or ask you to stop using it for a while or permanently, depending on the results of your blood and urine tests or the occurrence of side effects.
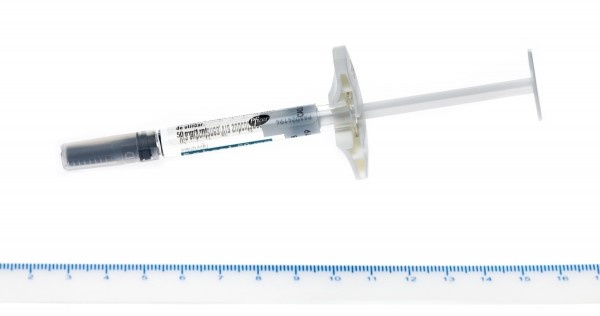
You (or your caregiver) will be taught how to use Waylivra by following the instructions in this leaflet. Waylivra should be injected under the skin (subcutaneously, or SC) as your doctor, nurse, or pharmacist has shown you, and check that all the liquid from the syringe has been injected.
Each single-dose pre-filled syringe of this medicine gives a dose of 285 mg in 1.5 ml.
Before using this medicine, it is important that you read, understand, and follow the administration instructions carefully.
The administration instructions are at the end of this leaflet.
If you use more Waylivra than you should
If you inject too much Waylivra, contact your doctor or pharmacist, or go to the emergency department immediately, even if you do not have any symptoms.
If you forget to use Waylivra
Do not take a double dose to make up for a forgotten dose. If you forget to administer a dose and realize it before 48 hours have passed since the scheduled time, administer the missed dose as soon as possible. However, if more than 48 hours have passed, you should wait until the next scheduled administration. You should not inject more than one dose in a 2-day period.
If you stop using Waylivra
Do not stop using Waylivra without talking to your doctor.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
If you get any of the following side effects, contact your doctor immediately:
- Symptoms that may indicate that the number of platelets in your blood is low (platelets are cells that are important for blood clotting). You should consult your doctor immediately if you have signs of a low platelet count, such as unusual or prolonged bleeding, red spots on the skin (called petechiae), unexplained bruising, bleeding that does not stop, nosebleeds, or if you have stiffness in the neck or a severe headache.
Other side effects
Very common(may affect more than 1 in 10 people)
- Reactions at the injection site (rash, pain, redness, heat, dryness, swelling, itching, tingling, hardening, hives, blisters, bumps, hematoma, bleeding, numbness, paleness, change in color, or a burning sensation at the injection site). You can reduce the likelihood of having a reaction at the injection site if you wait for Waylivra to reach room temperature before injecting it and apply ice to the injection site after doing so.
- Headache
- Muscle pain
- Chills
Common(may affect up to 1 in 10 people)
- Abnormally high levels of white blood cells in blood tests
- Abnormally low levels of white blood cells (known as lymphopenia) in blood tests
- Easily bruising or having unexplained bruising, or having bruises without an apparent cause
- Bleeding under the skin that looks like a rash, bleeding from the gums or mouth, blood in the urine or stool, nosebleeds, or having an abnormally heavy period
- Allergic reaction, whose symptoms may be skin rash, joint stiffness, or fever
- Blood or protein in the urine
- Changes in the results of certain blood tests, such as:
- increased levels of certain blood components: creatinine, urea, transaminases, liver enzymes
- increased coagulation time
- decreased hemoglobin concentration in the blood
- decreased glomerular filtration rate in the kidneys
- Diabetes, whose symptoms may be increased thirst, needing to urinate frequently (especially at night), extreme hunger, intense fatigue, and unexplained weight loss
- Difficulty sleeping
- Numbness, tingling, or prickling, feeling faint or dizzy, dizziness, or restlessness
- Vision disorders, such as flashes of light or brief transient blindness in one eye, bleeding under the surface of the eye, or blurred vision
- High blood pressure
- Feeling hot, increased sweating, night sweats, feeling of heat, flu-like illness, or general feeling of being unwell
- Cough, difficulty breathing, nasal congestion, throat swelling, wheezing
- Feeling sick, dry mouth, diarrhea, swelling of the neck, face, or gums, stomach pain or inflammation, indigestion
- Redness of the skin, rash, acne, thickening or scarring of the skin, or itching of the skin known as hives
- Pain in hands or feet, pain in the large joints of the arms and legs, including elbows, wrists, knees, and ankles, other types of pain or joint stiffness, back pain, neck pain, jaw pain, muscle spasms, or other body pains
- Intense fatigue, weakness, or lack of energy, fluid retention, chest pain not related to the heart.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Waylivra
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the syringe after "EXP". The expiry date is the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C).
Keep the pre-filled syringe in the outer carton in order to protect it from light.
Waylivra may be stored at room temperature (up to 30°C) in the original carton for up to 6 weeks after it is first removed from the refrigerator. During this time, and as needed, this medicine can be stored at room temperature or in the refrigerator. Record the date you first remove the carton from the refrigerator in the space provided on the carton. If you do not use it within the 6 weeks after first removing it from the refrigerator, discard the medicine. If, during the 6-week period that the syringe can be stored at room temperature, the expiry date on the label passes, do not use the syringe and discard it.
Do not use this medicine if you notice that the solution is cloudy or contains particles; it should be clear and colorless to pale yellow.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Waylivra Composition
- The active ingredient is volanesorsen. Each ml contains 200 mg of volanesorsen sodium, equivalent to 190 mg of volanesorsen. Each single-dose prefilled syringe contains 285 mg of volanesorsen in 1.5 ml of solution.
- The other ingredients are water for injectable preparations, sodium hydroxide, and hydrochloric acid (for acidity adjustment, see section 2, "Sodium").
Product Appearance and Container Contents
Waylivra is presented in a container that carries a single-dose syringe, with a needle and needle protector, prefilled with a clear, colorless to pale yellow solution. Filled to administer 1.5 ml of solution when the syringe plunger is fully pushed.
It is marketed in a container with a single prefilled syringe or in a multiple container with 4 (4 containers of 1 container) prefilled syringes.
Marketing Authorization Holder
Akcea Therapeutics Ireland Ltd.
St. James House
72 Adelaide Road, Dublin 2
D02 Y017
Ireland
Manufacturer
Almac Pharma Services Ireland Ltd.
Finnabair Industrial Estate
Dundalk
Co. Louth
Ireland
Date of Last Revision of this Leaflet: 11/2022
This medicinal product has been authorized with a "conditional approval". This approval modality means that more information is expected to be obtained about this medicinal product.
The European Medicines Agency will review the new information about this medicinal product at least once a year, and this leaflet or summary of product characteristics (SPC) will be updated as necessary.
Other Sources of Information
Detailed information about this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
Instructions for Use
Waylivra is an injection given under the skin with a prefilled syringe, disposable and for single use.
Do not use Waylivra until you understand the procedure described below. If you have any doubts about the use of Waylivra, contact your doctor or pharmacist.
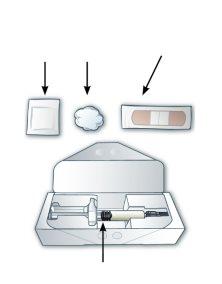
Prefilled Syringe Parts


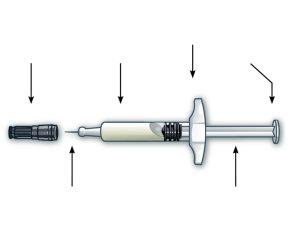
Preparation for Injection
- Wash your hands and gather what is necessary for the injection
Wash your hands thoroughly with soap (for at least 3 minutes) and dry them well.
Place the following items on a flat and clean surface, in a well-lit area (Figure A).
|
|
Figure A
- Let the injection reach room temperature
If the syringe is in the refrigerator, let it reach room temperature by taking it out of the refrigerator at least 30 minutes before the injection. If the injected liquid is cold, it may cause reactions at the injection site such as pain, redness, or swelling. Do notheat the syringe in any other way, neither in the microwave nor with hot water. |
Figure B |
- Check the expiration date
Check the expiration date on the box. The expiration date on the container refers to the life of the medicinal product when it is refrigerated. The first time you take the container out of the refrigerator, you should write the date in the space indicated on the box. Do notuse Waylivra if it has passed the expiration date or if it has been more than 6 weeks at room temperature. Contact your doctor or pharmacist to obtain new medication. |
- Remove the syringe and inspect the medication
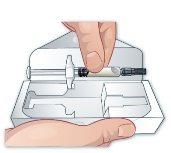
Open the box and remove the syringe, holding it by the cylinder and pulling it out (Figure C). |
Figure C |
Observe the liquid in the syringe. It should be clear to pale yellow. It is normal to see a large air bubble (Figure D). Do nottry to remove the bubble before the injection. It is okay if the solution is injected with the bubble. Do notuse the prefilled syringe if the liquid appears cloudy or has floating particles. |
Figure D |
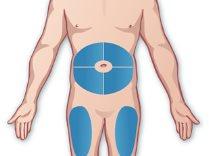
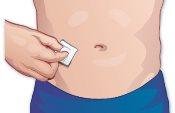
- Choose an injection site
If you are self-administering: Abdomen – The areas of the abdomen are shown, except for the 5 cm surrounding the navel. Thighs – The front and middle faces are shown (Figure E). |
Figure E |
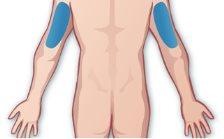
If a caregiver is administering the injection, in addition to these two areas, the injection can be given in: Arms – The back of the arm, as shown (Figure F). For all injections: Rotate the injection sites. Avoid injecting into the waist: clothing may rub or press the injection site. Do not injectinto tattoos, moles, scars, birthmarks, bruises, rashes, or areas of painful skin to the touch, red, hard, damaged, burned, or inflamed. If you are unsure where to inject, talk to your healthcare professional. |
Figure F |
Injection
- Prepare the injection site
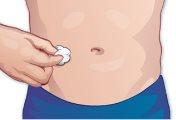
Clean the chosen area with a cotton ball soaked in alcohol (Figure G). |
Figure G |
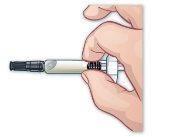
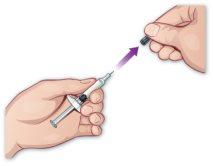
- Remove the syringe protector
Remove the syringe protector by holding the cylinder of the syringe without the needle pointing to you and pulling the protector off (Figure H). There may be a drop of liquid on the tip of the needle. This is normal. Do nothold the plunger or its support when removing the needle protector. Do notuse the prefilled syringe if the needle appears damaged. Do not usethe prefilled syringe if it falls after removing the needle protector. |
Figure H |
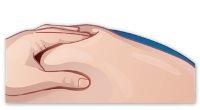
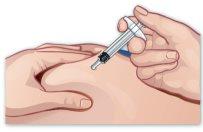
- Pinch the skin
Pinch the skin around the injection site with your free hand (Figure I). |
Figure I |
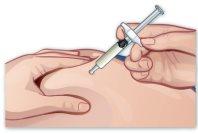
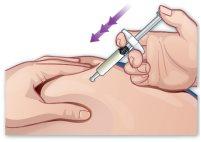
- Insert the needle
Insert the needle into the injection site with a quick and firm movement, without touching the plunger support. The needle should be inserted at a 45-degree angle to the skin surface (Figure J). |
Figure J |
- Inject Waylivra
Inject the liquid by holding the syringe with your thumb on the plunger and pushing it slowlyto the end of its path, until the syringe is completely empty (Figures K and L). |
Figure K |
Figure L |
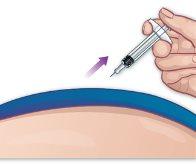
- Remove the needle
Remove the needle from the injection site by pulling it out at the same angle as it was inserted (Figure M). |
Figure M |
After the injection
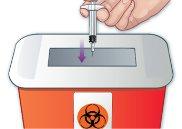
- Dispose of the used syringe in a sharp object container
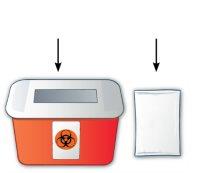
Immediately after the injection, dispose of the used syringe as your healthcare professional indicates, usually in containers for disposing of sharp objects (Figure N) following these steps. Discard the needle protector after the injection. Do notrecap the syringe. If you do not have a container for disposing of sharp objects, you can throw it away in a household container if:
|
Figure N |
When the container for disposing of sharp objects is almost full, you should follow local guidelines for safe disposal. There may be local regulations on how to dispose of used needles and syringes. Ask your pharmacist or check the website of the local public health authority (if applicable) for more information on how to dispose of sharp objects in your area. Do notthrow your container for disposing of sharp objects in the trash. Do notrecycle your used container for disposing of sharp objects. Always keep the container for sharp objects out of the reach of children and pets. |
- Treat the injection site
If blood comes out of the injection site, press the area gently with a sterile cotton ball or gauze, if necessary (Figure O). Do notrub the area after the injection. |
Figure O |
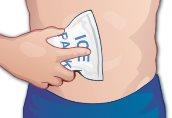
You can also apply ice to the injection site to reduce pain, redness, or discomfort (Figure P). |
Figure P |
Storage
Storage Information
The first time you are given Waylivra, you should store the prefilled syringes in their container in the refrigerator (between 2 °C and 8 °C). Waylivra can be stored at room temperature (between 8 °C and 30 °C) in the box protected from light for up to 6 weeks. During these 6 weeks, this medicinal product can be stored at room temperature or in the refrigerator. Do notfreeze the Waylivra prefilled syringe. Do notremove it from the container or remove the needle protector until you are ready for the injection. Discard this medicinal product immediately if it has not been used within 6 weeks of being removed from the refrigerator. To be sure, check the date you wrote on the box. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to WAYLIVRA 285 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: TABLET, 10 mg ezetimibeActive substance: ezetimibeManufacturer: Organon Salud S.L.Prescription requiredDosage form: CAPSULE, 1000 mgActive substance: omega-3-triglycerides incl. other esters and acidsManufacturer: Kern Pharma S.L.Prescription requiredDosage form: CAPSULE, 1000 mgActive substance: omega-3-triglycerides incl. other esters and acidsManufacturer: Strides Pharma (Cyprus) LimitedPrescription required
Online doctors for WAYLIVRA 285 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about WAYLIVRA 285 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions