
VENTOALDO 100 mcg/DOSIS SUSPENSION PARA INHALACION EN ENVASE A PRESION

Cómo usar VENTOALDO 100 mcg/DOSIS SUSPENSION PARA INHALACION EN ENVASE A PRESION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Ventoaldo 100 microgramos/dosis y para qué se utiliza
- Qué necesita saber antes de empezar a usar Ventoaldo 100 microgramos/dosis
- Cómo usar Ventoaldo 100 microgramos/dosis
- Posibles efectos adversos
- Conservación de Ventoaldo 100 microgramos/dosis
- Contenido del envase e información adicional
Introducción
Prospecto: información para el usuario
Ventoaldo 100 microgramos/dosis suspensión para inhalación en envase a presión
salbutamol
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted,y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Ventoaldo 100 microgramos/dosis y para qué se utiliza
- Qué necesita saber antes de empezar a usar Ventoaldo 100 microgramos/dosis
- Cómo usar Ventoaldo 100 microgramos/dosis
- Posibles efectos adversos
- Conservación de Ventoaldo 100 microgramos/dosis
- Contenido del envase e información adicional
1. Qué es Ventoaldo 100 microgramos/dosis y para qué se utiliza
El salbutamol pertenece al grupo de medicamentos denominados broncodilatadores que actúan relajando los músculos de las paredes de los pequeños conductos de aire de los pulmones. Facilita la respiración y alivia la tos.
- Tratamiento sintomático del broncoespasmo (cierre de conductos de aire en los pulmones) en el asma bronquial y en otros procesos asociados a obstrucción reversible de las vías respiratorias. Estas son un grupo de enfermedades pulmonares que causan inflamación de las vías respiratorias ocasionando un bloqueo en el flujo de aire en los pulmones.
- Prevención del broncoespasmo (cierre de conductos de aire en los pulmones) inducido por ejercicio físico o antes de exponerse a un estímulo alergénico (sustancia capaz de producir una reacción alérgica) conocido e inevitable.
2. Qué necesita saber antes de empezar a usar Ventoaldo 100 microgramos/dosis
No use Ventoaldo 100 microgramos/dosis:
- Si es alérgico al salbutamol o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar este medicamento:
- Si tiene historial de enfermedad cardiaca, ritmo cardíaco irregular o angina de pecho.
- Si padece tirotoxicosis, diabetes mellitus, alteraciones cardiovasculares graves o hipertensión (tensión arterial elevada).
- Si durante el tratamiento su situación se agravase aún más deberá acudir al médico, pues posiblemente pueda necesitar otro tratamiento.
Otros medicamentos y Ventoaldo 100 microgramos/dosis
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Es importante que informe a su médico si toma alguno de los siguientes medicamentos:
- Los beta-bloqueantes no cardioselectivos dado que están contraindicados en pacientes asmáticos. El propranolol y similares antagonizan los efectos del salbutamol.
- Durante el tratamiento con salbutamol es preferible no administrar imipramina, clorpromazina ni clordiacepóxido.
- Medicamentos que disminuyan el potasio sérico ya que si se administran conjuntamente los efectos pueden ser aditivos.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No se recomienda el uso de salbutamol durante el embarazo, sólo se administrará cuando a criterio médico el beneficio esperado para la madre es mayor que cualquier posible riesgo para el feto.
En caso de tener que ser administrado a una madre en periodo de lactancia, se recomienda sustituir la lactancia natural.
Conducción y uso de máquinas:
Aunque no son de esperar efectos sobre la capacidad para conducir y utilizar máquinas, usted deberá tener en cuenta la posibilidad de que se presenten calambres musculares y temblores.
Uso en deportistas
Este medicamento contiene salbutamol, que puede producir un resultado positivo en las pruebas de control del dopaje.
Ventoaldo 100 microgramos/dosis contiene etanol
Este medicamento contiene 3 mg de alcohol (etanol) en cada pulsación. La cantidad por pulsación de este medicamento es equivalente a menos de 1 ml de cerveza o 1 ml de vino. La pequeña cantidad de alcohol que contiene este medicamento no produce ningún efecto perceptible.
3. Cómo usar Ventoaldo 100 microgramos/dosis
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Recuerde tomar su medicamento.
Este medicamento se administra por vía inhalatoria.
Ventoaldo 100 microgramos/dosis se debe utilizar a demanda y no de forma regular.
Si su asma está activa (por ejemplo, tiene síntomas o crisis frecuentes, como dificultad para respirar que le dificulta hablar, comer o dormir, tos, sibilancias, opresión en el pecho o capacidad física limitada), debe informar inmediatamente a su médico, que le puede empezar a administrar un medicamento o aumentar la dosis de tratamiento, como un corticosteroide inhalado, para controlar su asma.
Informe a su médico lo antes posible si su medicamento parece no estar funcionando tan bien como de costumbre (por ejemplo, si necesita dosis más altas para aliviar sus problemas respiratorios o si su inhalador no le proporciona alivio durante al menos 3 horas), ya que su asma puede estar empeorando y usted puede necesitar un medicamento diferente.
Si utiliza Ventoaldo 100 microgramos/dosis más de dos veces por semana para tratar sus síntomas asmáticos, sin incluir el uso preventivo antes del ejercicio, esto indica un asma mal controlada y puede aumentar el riesgo de ataques de asma graves (empeoramiento del asma) que pueden tener complicaciones serias y pueden poner en riesgo su vida o incluso ser mortales. Se debe poner en contacto con su médico lo antes posible para revisar su tratamiento del asma.
Si utiliza a diario un medicamento contra la inflamación de sus pulmones, p.ej., un “corticosteroide inhalado”, es importante que siga utilizándolo con regularidad, aunque se sienta mejor.
La dosis recomendada es:
Adultos
Para aliviar el broncoespasmo agudo y tratar los episodios intermitentes de asma, puede administrarse una inhalación como dosis única, pudiendo incrementarse a dos inhalaciones en caso necesario. Si la respuesta es inadecuada, se pueden utilizar dosis superiores a dos inhalaciones. La dosis máxima recomendada es de dos inhalaciones, tres o cuatro veces al día.
Para prevenir el broncoespasmo inducido por el ejercicio físico, se deben administrar una o dos inhalaciones 15 minutos antes del mismo.
Se pueden administrar una o dos inhalaciones antes de un contacto previsto con alergenos.
Uso en edad avanzada:
Las mismas recomendaciones que para los adultos.
Uso en niños y adolescentes
La dosis recomendada para aliviar el broncoespasmo agudo en el tratamiento del asma episódico o para prevenir el asma inducido por el ejercicio es de una inhalación. Si la respuesta es inadecuada, pueden administrarse dosis mayores que una inhalación.
Es muy importante que siga las instrucciones indicadas por el médico.
Instrucciones para la correcta administración del preparado:
- Quite la tapa (fig. 1). En caso de que sea un inhalador nuevo o no se haya utilizado durante varios días, agitar el aerosol (fig. 2) y efectúe una pulsación para asegurar el buen funcionamiento del inhalador. En caso de que el inhalador se utilice regularmente pase a las instrucciones siguientes:
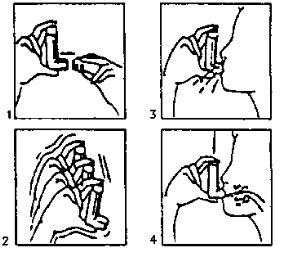 Agite el inhalador (fig. 2).
Agite el inhalador (fig. 2).- Elimine de sus pulmones la máxima cantidad de aire posible.
- Adapte el aerosol a su boca según la posición que se indica en el dibujo (fig. 3).
- Haga una inspiración lo más profunda posible.
Debe oprimir, según las flechas del dibujo (fig. 4), el aparato mientras está haciendo esta inspiración.
- Retire el aerosol de su boca y procure retener el aire en sus pulmones durante unos segundos.
- Debe lavarse periódicamente el pulsador-adaptador oral del aerosol. Para ello, retire el pulsador del aerosol y límpielo con un trapo o bien con un pañuelo de papel.H. Guarde con la tapa colocada y para protegerlo del polvo y de la suciedad.
El inhalador posee un indicador de dosis que se puede ver a través de un pequeño orificio o ventana del pulsador y que indica cuántas aplicaciones quedan. En este indicador de un inhalador nuevo se puede leer a través de la ventana del pulsador un “200”. Este número corresponde a la dosis que queda en el inhalador. A medida que se va utilizando el inhalador, el indicador de dosis va rotando de forma decreciente cada 5 - 7 pulsaciones hasta llegar a 0.
Cuando quedan aproximadamente 40 dosis el indicador cambia de verde a rojo (ver figura 5) con el fin de recordar al paciente que ha de consultar a su médico si ha de continuar el tratamiento o si necesita una nueva receta. Desechar el inhalador una vez el indicador llegue a “0”.

(figura 5)
Si usa más Ventoaldo 100 microgramos/dosis del que debe
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica. Teléfono 91 562 04 20. indicando el medicamento y la cantidad ingerida.
En caso de tomar una dosis mayor de la recomendada, puede aparecer vasodilatación periférica, aumento del número de pulsaciones y temblor del músculo esquelético. Esta sintomatología desaparece rápidamente de modo espontáneo. Para anular los efectos de la beta-estimulación adrenérgica pueden usarse fármacos beta-bloqueantes cardioselectivos (practolol). Otros beta-bloqueantes adrenérgicos no se recomiendan, ya que pueden producir broncoconstricción en asmáticos. En el caso de que se presentara, en la intoxicación aguda, arritmia ventricular, se recomienda la infusión intravenosa lenta de cloruro potásico, 40 mEq en 500 ml de dextrosa inyectable al 5%.
Si olvidó usar Ventoaldo 100 microgramos/dosis
No tome una dosis doble para compensar las dosis olvidadas. Inhalar la próxima dosis cuando corresponda o antes en caso de notar ahogo o “pitidos”.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos son dosis dependientes y son debidos al mecanismo de acción de los beta-2 agonistas.
Aunque no se conoce exactamente con qué frecuencia ocurre, algunas personas pueden experimentar ocasionalmente dolor en el pecho (debido a problemas de corazón tales como angina de pecho). Avise a su médico si desarrolla estos síntomas mientras está siendo tratado con salbutamol, pero no deje de tomar este medicamento a menos que así lo indique su médico.
En muy raras ocasiones, se han comunicado reacciones de alergia, que incluyen angioedema (inflamación de las capas profundas de la piel que se manifiesta a modo de grandes ronchas alrededor de los ojos y labios, pudiendo afectar también las manos, pies y garganta) y urticaria, broncoespasmo, hipotensión (descenso de la tensión arterial) y desmayo.
Trastornos en el aparato circulatorio y linfático: puede producirse hipocalemia potencialmente grave como consecuencia del tratamiento sistémico de agonistas beta-2.
Trastornos psiquiátricos: nerviosismo, sensación de tensión. Al igual que con otros agonistas beta-2, rara vez se ha comunicado hiperactividad en niños.
Trastornos del sistema nervioso: temblor leve, dolor de cabeza, mareos.
Trastornos cardiovasculares: taquicardia, angioedema, hipotensión. Se han comunicado casos de arritmias cardíacas (incluyendo fibrilación auricular, taquicardia supraventricular y extrasístole) en asociación con los agonistas beta-2, normalmente en pacientes susceptibles.
Trastornos respiratorios, torácicos y mediastínicos: al igual que con otras terapias de inhalación, deberá tenerse en cuenta la posibilidad de broncoespasmo paradójico con un aumento inmediato en las sibilancias después de la administración.
Trastornos gastrointestinales: náuseas.
Trastornos cutáneos y subcutáneos: urticaria.
Trastornos del aparato locomotor, del tejido conjuntivo y óseos: rara vez se han comunicado casos de calambres musculares transitorios.
Trastornos en general y afecciones en el lugar de administración:pueden darse casos de irritación bucal y faríngea.
Si se observa cualquier otra reacción adversa no descrita anteriormente, consulte a su médico o farmacéutico.
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https//www.notificaram.es Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Ventoaldo 100 microgramos/dosis
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 30ºC. Conservar en el embalaje original para protegerlo de la luz. No congelar.
El envase contiene un líquido a presión. No exponer a temperaturas superiores a 50ºC. No perforar el envase aun cuando aparentemente esté vacío.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y estuche después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Ventoaldo 100 microgramos/dosis
-El principio activo es salbutamol base (equivalente a 120 microgramos de salbutamol sulfato)
-Los demás componentes (excipientes) son ácido oleico, etanol y Norflurano .
Aspecto del producto y contenido del envase
Ventoaldo 100 microgramos/dosis se presenta en forma de suspensión en envase a presión con indicador de dosis; cada caja contiene un envase de 10 ml que permite realizar 200 aplicaciones.
Titular de la autorización de comercialización y responsable de la fabricación
Laboratorio Aldo-Unión, S.L.
Baronesa de Maldá, 73
08950 Esplugues de Llobregat (Barcelona)
España
Fecha de la última revisión de este prospecto:
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a VENTOALDO 100 mcg/DOSIS SUSPENSION PARA INHALACION EN ENVASE A PRESIONForma farmacéutica: INHALACIÓN PULMONAR, 0.12% P/V SALBUTAMOL SULFATOPrincipio activo: salbutamolFabricante: Laboratorio Aldo Union S.L.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 0.24% P/V SALBUTAMOL SULFATOPrincipio activo: salbutamolFabricante: Laboratorio Aldo Union S.L.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 100 microgramos/aplicaciónPrincipio activo: salbutamolFabricante: Laboratorio Aldo Union S.L.Requiere receta
Médicos online para VENTOALDO 100 mcg/DOSIS SUSPENSION PARA INHALACION EN ENVASE A PRESION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de VENTOALDO 100 mcg/DOSIS SUSPENSION PARA INHALACION EN ENVASE A PRESION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















