
VACUNA BCG 0,75 mg/ml POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE

Cómo usar VACUNA BCG 0,75 mg/ml POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
PROSPECTO: INFORMACIÓN PARA EL USUARIO
Vacuna BCG 0,75 mg/ml polvo y disolvente para suspensión inyectable
Mycobacterium bovis
Lea todo el prospecto detenidamente antes deque usted o su hijo sean vacunado, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted o a su hijo y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es la Vacuna BCG y para qué se utiliza
- Qué necesita saber antes de recibir usted o su hijo la Vacuna BCG
- Cómo se administra la Vacuna BCG
- Posibles efectos adversos
- Conservación de la Vacuna BCG
- Contenido del envase e información adicional
1. Qué es la Vacuna BCG y para qué se utiliza
La vacuna BCG polvo y disolvente para suspensión inyectable pertenece al grupo de medicamentos denominados vacunas antituberculosas.
La vacuna BCG está indicada para la prevención de la tuberculosis. Aunque no asegura una completa inmunidad, aumenta la resistencia a la infección tuberculosa.
La vacuna BCG debe usarse en base a las recomendaciones oficiales.
2. Qué necesita saber antes de recibirusted o su hijo la Vacuna BCG
Nousela Vacuna BCG
- Si es alérgico (hipersensible) a Mycobacterium boviso a alguno de los demás componentes de la vacuna BCG (incluidos en la sección 6).
- Si padece tuberculosis o cualquier otra enfermedad infecciosa (activa o durante su convalecencia) o si está en tratamiento antituberculosis.
- Si padece algún trastornos de inmunidad, fundamentalmente en pacientes con infección por VIH, en niños nacidos de madres seropositivas, en casos de inmunodeficiencia congénita, o casos con la respuesta inmune disminuida por la acción de ciertos medicamentos (corticoides, agentes alquilantes, antimetabolitos) o la radiación.
- Si ha sido expuesto a un tratamiento inmunosupresor en el útero o durante la lactancia (por ejemplo tratamiento con un antagonista α-FNT).
- Si su estado inmunitario es cuestionable.
- En niños con malnutrición Kwashiorkor (que cursa con una deficiencia de proteínas y calorías).
- Si padece síndrome febril agudo severo o alguna enfermedad generalizada en la piel (se debe posponer la vacunación).
- Si padece angiopatías (enfermedades de los vasos sanguíneos) o hemopatías (enfermedades de la sangre) graves.
- Si padece procesos oncológicos.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de que usted o su hijo sean vacunados con la vacuna BCG.
Antes de iniciar el tratamiento con la Vacuna BCG, deberán realizarle la prueba de la tuberculina. Hasta los ocho años de edad se pueden utilizar las pruebas en la piel, pero en niños mayores o adultos, debe emplearse la prueba intracutánea de Mantoux con Tuberculina.
Aunque las reacciones alérgicas son raras, se debe contar con medidas necesarias para su tratamiento y, si es posible, se recomienda observar al paciente hasta 15-20 minutos posteriores tras la inyección en busca de síntomas de reacción alérgica.
En caso de que el paciente padezca eccema, la inyección de la vacuna BCG no está contraindicada, pero debe realizarse la inyección en una zona libre de lesiones.
Uso dela Vacuna BCG conotros medicamentos
Informe a su médico o farmacéutico si usted o su hijo está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
No se debe administrar la vacuna a pacientes que hayan sido tratados con medicamentos antituberculosos.
La vacuna BCG puede ser administrada al mismo tiempo que vacunas vivas, incluyendo las combinadas (sarampión, paperas y rubéola), teniendo especial precaución para no administrarlas en el mismo brazo. En caso de que la administración no sea simultánea, debe dejarse un intervalo de 4 semanas entre la administración de las dos vacunas vivas.
Para evitar el riesgo de hinchazón y dolor en los ganglios linfáticos de la zona, se recomienda no usar el mismo brazo en el que se ha aplicado la vacuna BCG para la administración de otras vacunas durante un período de tres meses.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de ser vacunada con la Vacuna BCG.
Aunque no se han asociado daños al feto con el uso de la Vacuna BCG, no se recomienda su administración durante el embarazo o lactancia, a menos que exista un riesgo excesivo o inevitable de exposición al contagio de la tuberculosis.
Consulte a su médico o farmacéutico antes de utilizar un medicamento.
Conducción y uso de máquinas
La Vacuna BCG no afecta a su capacidad de conducir o manejar maquinaria
La Vacuna BCG contiene de sodio
Este medicamento contiene menos de 23 mg de sodio por dosis; esto es esencialmente “exento de sodio”.
3. Cómo se administra la Vacuna BCG
Siga exactamente las instrucciones de administración de la Vacuna BCG indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendad es:
Adultos y niños mayores de 1 año: una dosis única de 0,1 ml.
Niños menores de 1 año: una dosis única de 0,05 ml.
Forma de uso y vía de administración
La Vacuna BCG se administra estrictamente por VÍA INTRADÉRMICA en la cara externa superior del brazo y en la cara externa del muslo. La inyección debe realizarse lentamente en la capa superior de la piel por personal entrenado, dado que si la inyección se realiza más profundamente, se aumenta el riesgo de formación de abscesos (acúmulos localizados de pus en la piel).
Si usa másVacuna BCGde la que debiera
En casos de sobredosis, sobre todo en niños pequeños, puede presentarse linfadenitis (inflamación de los ganglios linfáticos) supurativa benigna que se cura de forma lenta y espontánea.
En casos excepcionales puede desarrollarse una infección generalizada por la vacuna BCG. Se debe buscar consejo respecto al régimen de tratamiento para el manejo de infecciones sistémicas o infecciones locales persistentes tras la vacunación con la vacuna BCG.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica (Teléfono: 91 562 04 20) indicando el medicamento y la cantidad ingerida.
Si omitió la administración de la Vacunabcg
Como dosis única, es poco probable que se olvide su dosis. No obstante, comunique a su médico o farmacéutico si ha omitido su dosis.
4. Posibles efectos adversos
Al igual que todos los medicamentos, la Vacuna BCG puede producir efectos adversos aunque no todas las personas los sufran.
En general, esta vacunación no suele causar fiebre o malestar. Algunos días después de la vacunación se desarrolla un nódulo de induración (abultamiento endurecido de tejido que se forma en la piel) en el sitio de la inyección. Este nódulo disminuye gradualmente y es reemplazado por una lesión local que puede ulcerarse algunas semanas más tarde. La lesión local no requiere tratamiento ni deben utilizarse apósitos. Esta lesión cura espontáneamente con formación de una pequeña costra negruzca.
Ocasionalmente, puede observarse un engrosamiento de los nódulos linfáticos, cervicales o axilares, que tampoco requiere tratamiento.
Se han observado las siguientes reacciones adversas clasificadas por órganos y sistemas organizados en orden decreciente según su frecuencia de aparición:
Efectos adversos poco frecuentes (al menos 1 de cada 1.000 pacientes):
Aumento de tamaño de los ganglios linfáticos (> 1 cm), dolor de cabeza, fiebre, úlcera en la zona de inyección, inflamación con pus de los ganglios.
Efectos adversos raros (al menos 1 de cada 10.000 pacientes):
Infección diseminada, tal como inflamación aguda o crónica de los huesos, originada o no por una infección, absceso en la zona de inyección, reacción alérgica, reacción de hipersensibilidad.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de la Vacuna BCG
Conservar en nevera (entre 2ºC y 8ºC) protegido de la luz.
Utilizar únicamente en las 4 horas siguientes a su reconstitución. Una vez superado ese plazo, desechar la suspensión.
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice la Vacuna BCG después de la fecha de caducidad que aparece en el envase. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de la Vacuna BCG
- El principio activo de la Vacuna BCG es Mycobacterium bovis(BCG) Cepa Danesa 1331. Cada 1 ml de vacuna reconstituida contiene 0,75 mg de Mycobacterium bovis(BCG) Cepa Danesa 1331, con 2-8 x106 UFC/ml.
- Los demás componentes son: glutamato de sodio, sulfato de magnesio, fosfato dibásico de potasio, L-asparagina monohidratado, citrato férrico-amónico, glicerol 85%, ácido cítrico monohidratado y agua para preparaciones inyectables, c.s.
Aspecto del producto y contenido del envase
La vacuna BCG se presenta en forma de polvo y disolvente para suspensión inyectable.
El polvo es un liofilizado blanco cristalino, difícilmente perceptible a la vista por la escasa cantidad que contiene el vial. El polvo se acondiciona en un vial topacio de vidrio tipo I con tapón de bromobutilo y cápsula de aluminio.
El disolvente es una solución incolora sin partículas visibles. El disolvente se acondiciona en un vial de vidrio tipo I con tapón de clorobutilo y cápsula de aluminio.
Un vial de la Vacuna BCG reconstituida contiene 1 ml, correspondiente a 10 dosis para adultos y niños mayores de 1 año (0,1 ml) o a 20 dosis para niños menores de 1 año (0,05 ml).
Titular de la Autorización de Comercialización y Responsable de la Fabricación
AJ Vaccines A/S, Artillerivej 5. DK-2300 Copenhague S, Dinamarca.
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
MEDICARE PHARMA, S.L.
Paseo de la Castellana, 177 3ºB
28046 Madrid, España
Este prospecto fue aprobado en 06/2020
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
_____________________________________________________________________________________
Esta información está destinada únicamente a profesionales del sector sanitario:
Advertencias especiales y precauciones de uso
La vacuna se debe administrar únicamente por vía intradérmica.
Preferentemente, la vacuna debe ser administrada por personal entrenado en la técnica de vacunación intradérmica.
Las inyecciones administradas de forma inadecuada, por ejemplo por via subcutñanea o intramuscular aumentan el riesgo de linfadenitis y de formación de abscesos
No se debe vacunar a las personas que den positivo en el test de la tuberculina ya que esto puede producir un empeoramiento de la reacción loco-regional.
Aunque las reacciones anafilácticas son raras, durante la vacunación se debe disponer de instalaciones para su tratamiento.
Siempre que sea posible, las personas se deben mantener en observación durante 15–20 minutos tras la vacunación en caso de que se produjera una reacción alérgica.
La vacuna BCG puede ser administrada al mismo tiempo que vacunas inactivadas o vivas, incluyendo vacunas combinadas contra sarampión, paperas y rubéola. Si no se administran simultáneamente, se debe dejar un intervalo mínimo de 4 semanas antes de administrar otra vacuna viva.
Se debe esperar un intervalo mínimo de 3 meses antes de poner una nueva vacuna en el mismo brazo.
Manejo
El tapón de goma no debe ser limpiado con ningún antiséptico o jabón. Si se utiliza alcohol para limpiar el tapón del vial, hay que dejar que se evapore antes de que la aguja de la jeringa lo atraviese.
Usando una jeringa equipada con una larga aguja, transferir al vial el volumen de disolvente especificado en la etiqueta.
No utilice otros disolventes ya que podrían estropear la vacuna.
Invertir el vial cuidadosamente varias veces para resuspender el liofilizado completamente.
NO AGITAR. Antes de la extracción de cada dosis de vacuna resuspendida, agitar suavemente el vial.
Cuando se extraiga en la jeringa, la suspensión de vacuna debe parecer homogénea, ligeramente opaca e incolora.
Una vez reconstituida la vacuna debe usarse en las siguientes 4 horas.
Método de administración
La Vacuna BCG debe ser administrada por personal entrenado en la técnica intradérmica.
El lugar donde va a aplicarse la inyección debe estar limpio y seco.
Si se utiliza un antiséptico (por ejemplo, alcohol) para limpiar la piel, hay que dejar que se evapore completamente antes de la inyección.
La Vacuna BCG se administra estrictamente por VIA INTRADÉRMICA en el tercio superior del brazo correspondiente al área de inserción distal del músculo deltoides de la forma siguiente:
- Debe estirarse la piel entre el dedo índice y pulgar.
- La aguja debe estar casi paralela a la superficie de la piel e insertarse lentamente (con el bisel hacia arriba), aproximadamente 2 mm en la capa superficial de la dermis. La aguja debe ser visible a través de la epidermis durante la inserción.
- La inyección debe realizarse lentamente.
- Si la administración es correcta, aparecerá una pápula blanquecina en el punto de inyección.
- Se recomienda no proteger el punto de inyección para facilitar la cicatrización.
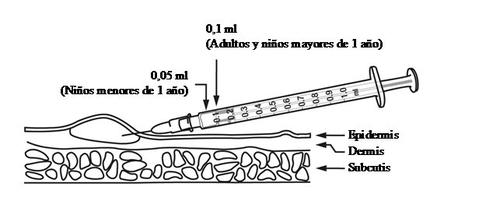
La Vacuna BCG debe administrase con una jeringa de 1 ml graduada en centésimas de ml (1/100 ml) equipada con una aguja de bisel corto de calibre 25G ó 26 G. No deben utilizarse para la administración de esta vacuna inyectores a presión ni dispositivos de punción múltiple.
Sobredosis o administración incorrecta
Una sobredosis aumenta el riesgo de linfadenitis supurativa y puede producir una formación excesiva de escaras.
Una sobredosis masiva aumenta el riesgo de efectos adversos de la Vacuna BCG.
La inyección profunda de la vacuna incrementa el riesgo de ulcera supurante, linfadenitis y de formación de abscesos
Tratamiento de las complicaciones tras la vacunación con la Vacuna BCG
Se debe buscar consejo respecto del apropiado régimen de tratamiento para el manejo de infecciones sistémicas o infecciones locales persistentes tras la vacunación con la Vacuna BCG.
Sensibilidad de la cepa BCG frente a antibióticos:
La tabla inferior indica los valores de concentración mínima inhibitoria (CMI) para los varios medicamentos antituberculosos seleccionados frente a la cepa Danesa 1331 [determinados con el método Bactec 460].
La CMI para isoniacida es 0,4 mg/l. No hay consenso sobre si Mycobacterium bovis debe ser clasificado como susceptible, intermedio o resistente a la isoniacida cuando la CIM es 0,4 mg/l. Sin embargo, en base al criterio establecido para Mycobacterium tuberculosis, la cepa se considera que es de susceptibilidad intermedia.
Medicamento | Concentración Mínima Inhibitoria (CMI) |
Isoniazida | 0,4 mg/l |
Estreptomicina | 2,0 mg/l |
Rifampicina | 2,0 mg/l |
Etambutol | 2,5 mg/l |
La Cepa Danesa 1331 es resistente a la pirazinamida.
- País de registro
- Precio medio en farmacia78.05 EUR
- Disponibilidad en farmacias
Problema de suministro reportado
Los datos de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) indican un problema de suministro que afecta a este medicamento.<br><br>La disponibilidad puede ser limitada en algunas farmacias.<br><br>Para actualizaciones o alternativas, consulte a su farmacéutico. - Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a VACUNA BCG 0,75 mg/ml POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLEForma farmacéutica: INYECTABLE, 0,5 ml dosis únicaPrincipio activo: meningococcus B, multicomponent vaccineFabricante: Glaxosmithkline Vaccines S.R.L.Requiere recetaForma farmacéutica: INYECTABLE, 0,5 ml dosis únicaPrincipio activo: meningococcus B, multicomponent vaccineFabricante: Glaxosmithkline Vaccines S.R.L.Requiere recetaForma farmacéutica: INYECTABLE, -Principio activo: pertussis, purified antigen, combinations with toxoidsFabricante: Glaxosmithkline S.A.Requiere receta
Médicos online para VACUNA BCG 0,75 mg/ml POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de VACUNA BCG 0,75 mg/ml POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















