TYSABRI 150 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar TYSABRI 150 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Tysabri 150mg solución inyectable en jeringa precargada
Natalizumab
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
Además de este prospecto, se le entregará una tarjeta de información para el paciente y, en caso de autoadministración o administración por un cuidador, una Lista de verificación previa a la administración. Estas contienen información importante sobre seguridad que debe conocer antes de recibir y durante el tratamiento con Tysabri.
- Conserve este prospecto y la tarjeta de información para el paciente, ya que puede tener que volver a leerlos. Conserve el prospecto y la tarjeta de información para el paciente con usted durante el tratamiento y durante seis meses después de la última dosis de este medicamento, ya que se pueden producir efectos adversos incluso después de haber interrumpido el tratamiento.
Si usted o su cuidador administran el tratamiento, revisen la Lista de verificación previa a la administración antes de cada dosis.
- Si tiene alguna duda, consulte a su médico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Tysabri y para qué se utiliza
- Qué necesita saber antes de empezar a recibir Tysabri
- Cómo se administra Tysabri
- Posibles efectos adversos
- Conservación de Tysabri
- Contenido del envase e información adicional
1. Qué es Tysabri y para qué se utiliza
TYSABRI se usa para tratar la esclerosis múltiple (EM). Contiene el principio activo natalizumab. Es lo que se llama un anticuerpo monoclonal.
La EM causa una inflamación en el cerebro que daña las células nerviosas. Esta inflamación se produce cuando los glóbulos blancos llegan al cerebro y a la médula espinal. Este medicamento impide que los glóbulos blancos lleguen al cerebro. Esto reduce la lesión nerviosa causada por la EM.
Síntomas de la esclerosis múltiple
Los síntomas de la EM pueden variar de un paciente a otro; es posible que usted experimente algunos o ninguno.
Pueden incluir: problemas para caminar, hormigueo en la cara, los brazos o las piernas; problemas de visión; cansancio; sensación de inestabilidad o mareo; problemas vesicales e intestinales; dificultad para pensar y concentrarse; depresión; dolor agudo o crónico; problemas sexuales; rigidez y espasmos musculares.
Cuando los síntomas se recrudecen, se denomina recidiva(también exacerbación o brote). Cuando se produce una recidiva, es posible que advierta los síntomas súbitamente, en el plazo de unas horas, o con una progresión lenta durante varios días. Los síntomas generalmente mejoran de forma gradual (esto se denomina remisión).
Cómo puede ayudar Tysabri
En ensayos, este medicamento redujo aproximadamente a la mitad el aumento de la discapacidad causada por la EM y redujo el número de brotes de EM en, aproximadamente, dos tercios. Mientras esté en tratamiento con este medicamento, es posible que no note ninguna mejoría, pero puede seguir actuando para prevenir el empeoramiento de la EM.
2. Qué necesita saber antes de empezar a recibir Tysabri
Antes de comenzar el tratamiento con este medicamento, es importante que usted y su médico hayan discutido los efectos beneficiosos que cabe esperar recibir de este tratamiento y los riesgos asociados al mismo.
No le deben administrar Tysabri
- Si es alérgico a natalizumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Si le han diagnosticado leucoencefalopatía multifocal progresiva(LMP). La LMP es una enfermedad poco frecuente del cerebro.
- Si su sistema inmunitariotiene un problema grave. Esto puede deberse a una enfermedad (como infección por el VIH) o a medicamentos que esté usando o que haya usado en el pasado (ver más adelante).
- Si está tomando medicamentos que afectan al sistema inmunitario, incluidos determinados medicamentos que se usan para el tratamiento de la EM. Estos medicamentos no pueden usarse con Tysabri.
- Si padece cáncer(a menos que se trate de un tipo de cáncer de piel llamado carcinoma basocelular).
Advertencias y precauciones
Consulte a su médicosi Tysabri es el tratamiento más adecuado para usted. Haga esto antes de empezar a utilizar este medicamento y cuando haya estado recibiéndolo durante más de dos años.
Llevar un registro
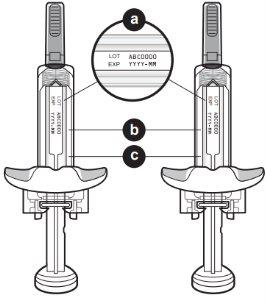
Con objeto de mejorar la trazabilidad de este medicamento, su médico o farmacéutico deben registrar el nombre y el número de lote del medicamento que se le ha administrado en su historia clínica. Usted también puede anotar estos datos por si se los piden en el futuro.
Posible infección cerebral (LMP)
Algunas personas que reciben este medicamento (menos de 1 de cada 100) han tenido una infección cerebral poco frecuente llamada LMP (leucoencefalopatía multifocal progresiva). La LMP puede provocar una discapacidad grave o la muerte.
- Antes de comenzar el tratamiento, el médico realizará un análisis de sangre a todos los pacientespara detectar la infección por el virus JC. El virus JC es un virus común que normalmente no hace que esté enfermo. Sin embargo, la LMP está relacionada con un aumento del virus JC en el cerebro. La razón de este aumento en algunos pacientes tratados con Tysabri no está clara. Antes y durante el tratamiento, su médico le hará un análisis de sangre para comprobar si tiene anticuerpos contra el virus JC (anticuerpos anti-VJC), que son un signo de que se ha infectado por el virus JC.
- Su médico realizará una exploración por resonancia magnética (RM), que se repetirá durante el tratamiento para descartar la LMP.
- Los síntomas de la LMPpueden ser similares a los de una recidiva de la EM (consulte la sección 4, Posibles efectos adversos). También puede presentar LMP hasta 6 meses después de suspender el tratamiento con Tysabri.
Informe a su médico lo antes posiblesi nota que su EM está empeorando o si advierte algún síntoma nuevo mientras está en tratamiento con Tysabri o hasta 6 meses después.
- Comunique a su pareja o cuidadoresqué deben tener en cuenta (consulte también la sección 4, Posibles efectos adversos). Algunos síntomas pueden ser difíciles de detectar por sí mismos, como cambios de humor o de comportamiento, confusión, dificultades del habla y de la comunicación. Si presenta alguno de ellos, es posible que le tengan que realizar más pruebas. Siga atento a los síntomas durante los 6 meses posteriores a la interrupción de Tysabri.
- Conserve la tarjeta de información para el paciente que su médico le ha entregado. Incluye esta información. Muéstresela a su pareja o cuidadores.
- Si usted o su cuidador administran el tratamiento, revisen la Lista de verificación previa a la administración antes de cada dosis.
Tres cosas pueden aumentar el riesgo de LMPcon Tysabri. Si tiene dos o más de estos factores de riesgo, el riesgo aumenta aún más:
- Si tiene anticuerpos anti-VJCen la sangre. Son un signo de que el virus está en su organismo. Se le realizarán pruebas antes y durante el tratamiento con Tysabri.
- Si está recibiendo un tratamiento prolongadocon Tysabri, especialmente si es durante más de dos años.
- Si ha tomado un medicamento llamadoinmunodepresor, que disminuye la actividad del sistema inmunitario.
El virus JC causa también otra afección, denominada NCG por VJC (neuronopatía de células granulares por virus JC) que se ha producido en algunos pacientes que reciben este medicamento. Los síntomas de la NCG por VJC son similares a los síntomas de la LMP.
En el caso de pacientes con menor riesgo de LMP, es posible que su médico repita los análisis periódicamente para comprobar:
- Si todavía no tiene anticuerpos anti-VJC en la sangre.
- Si ha recibido tratamiento durante más de 2 años, si todavía tiene un nivel más bajo de anticuerpos anti-VJC en la sangre.
Si alguien presenta LMP
La LMP puede tratarse y el tratamiento con Tysabri se interrumpirá. Sin embargo, algunas personas presentan una reaccióncuando Tysabri se elimina del organismo. Esta reacción (conocida como SIRIo síndrome inflamatorio de reconstitución inmune) puede hacer que su estado empeore, incluido un deterioro de la función cerebral.
Esté atento a otras infecciones
Algunas infecciones distintas de la LMP también pueden ser graves y pueden deberse a virus, bacterias y otras causas.
Informe al médico o enfermero inmediatamentesi cree que tiene una infección (ver también sección 4, Posibles efectos adversos).
Cambios en el número de plaquetas en sangre
Natalizumab puede reducir el número de plaquetas en la sangre, las cuales son responsables de la coagulación. Esto puede dar lugar a un trastorno llamado trombocitopenia (ver sección 4) por el que puede que su sangre no se coagule lo suficientemente rápido como para detener el sangrado. Esto puede provocar la aparición de moratones, así como otros problemas más graves como sangrado excesivo. Informe a su médico inmediatamente si presenta moratones inexplicables, manchas rojas o moradas en la piel (llamadas petequias), sangrado por cortes en la piel que no se detiene o supura, sangrado prolongado de las encías o de la nariz, sangre en la orina o en las heces o sangrado en la parte blanca de los ojos.
Niños y adolescentes
No administre este medicamento a niños o adolescentes menores de 18 años de edad.
Otros medicamentos y Tysabri
Informe a su médico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
- No le deben administrar este medicamentosi actualmente está siendo tratado con medicamentos que afectan a su sistema inmunitario, como determinados medicamentos para el tratamiento de la EM.
- Es posible que no pueda utilizar este medicamento si ha recibido alguna vezalgún medicamento que afecta al sistema inmunitario.
Embarazo y lactancia
- No utilice este medicamento si está embarazada, a menos que lo haya comentado con su médico. Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, debe informar inmediatamente a su médico.
- No dé el pecho mientras esté usando Tysabri.Su médico le ayudará a decidir si debe elegir dejar de dar el pecho o dejar de utilizar el medicamento.
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento. Su médico tendrá en cuenta el riesgo para el bebé y el beneficio para la madre.
Conducción y uso de máquinas
Los mareos son un efecto adverso muy frecuente. Si experimenta este síntoma, no conduzca ni utilice máquinas.
Tysabri contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis de 300 mg; esto es, esencialmente “exento de sodio”.
3. Cómo se administra Tysabri
Las inyecciones de Tysabri se las recetará un médico que tenga experiencia en el tratamiento de la EM. Su médico puede cambiarle directamente de otro medicamento a Tysabri si no hay signos de problemas causados por el tratamiento anterior.
- Su médico solicitará análisis de sangrepara detectar anticuerpos anti-VJC y otros posibles problemas.
- Su médico realizará una exploración por RM, que se repetirá durante el tratamiento.
- Para cambiar de algunos medicamentos para la EM, su médico puede recomendarle que espere un cierto tiempo para asegurarse de que la mayor parte del medicamento anterior se haya eliminado de su organismo.
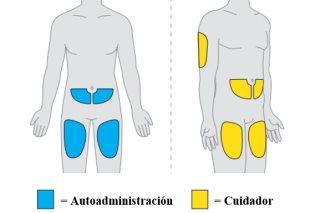
- Si su estado lo permite, su médico puede valorar con usted la opción de recibir las inyecciones fuera de un centro hospitalario (p. ej., en casa). Estas inyecciones se las puede administrar un profesional sanitario, usted mismo o un cuidador, siempre que cumpla determinados criterios. Seguirá teniendo que acudir al centro médico o al hospital para las citas, incluidas las de los análisis de sangre periódicos y las resonancias magnéticas.
- Si su médico decide que usted es apto para la autoadministración (o la administración por su cuidador), un profesional sanitario le supervisará durante la administración de las dos primeras dosis (2 inyecciones cada una).
- El profesional sanitario le dará a usted o a su cuidador instrucciones detalladas y le mostrará cómo preparar e inyectar el medicamento antes de utilizar las jeringas por primera vez.
- Si su médico decide que usted es apto para que se administre usted mismo o un cuidador, asegúrese de leer la tarjeta de información para el paciente para revisar la lista de síntomas de LMP y de revisar la Lista de verificación previa a la administración antes de cada dosis. Si aparece o empeora algún síntoma, no administre la dosis y póngase en contacto con su médico inmediatamente.
- La dosis recomendada para adultos es 300 mg una vez cada cuatro semanas.
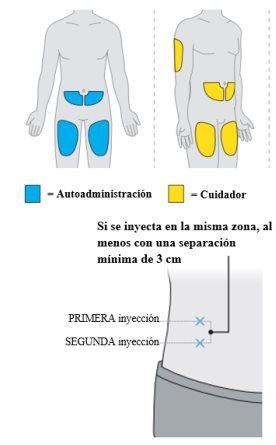
- Cada dosis se administra en dos inyeccionesdebajo de la piel, en el muslo, el abdomen (al menos a 6 centímetros del ombligo) o la parte posterior del brazo (únicamente en caso de inyección por un profesional sanitario o un cuidador). La administración requiere hasta 30 minutos.
- Al final del prospecto se proporciona información sobre cómo preparar e inyectar el medicamento.
Si interrumpe el tratamiento con Tysabri
Es importante la administración continua de Tysabri, especialmente durante los primeros meses de tratamiento. Es importante que continúe el tratamiento mientras usted y su médico decidan que le está ayudando. No deje de tomar su medicamento sin la recomendación de su médico. Los pacientes que recibieron una o dos dosis de Tysabri y luego hicieron una pausa en el tratamiento de 3 meses o más tuvieron una mayor probabilidad de sufrir una reacción alérgica al reanudar el tratamiento.
Comprobación de reacciones alérgicas
Algunos pacientes han presentado una reacción alérgica a este medicamento. Su médico comprobará si se producen reacciones alérgicas durante las inyecciones y durante 1 hora después. En caso de autoadministración o administración por un cuidador, si presenta una reacción alérgica interrumpa la inyección y acuda al médico inmediatamente. Ver también sección 4, Posibles efectos adversos.
Si olvidó usar Tysabri
Si no recibió su dosis habitual de Tysabri, hable con su médico para que se la administre lo antes posible. Después, puede seguir recibiendo Tysabri cada 4 semanas.
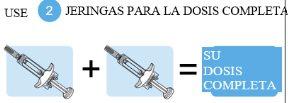
Es necesario administrar dos jeringas para proporcionar la dosis completa. Es importante que se administren ambas jeringasy que siga la pauta de administración prescrita. Si usted o su cuidador administran las inyecciones y han omitido una dosis o han inyectado solo una jeringa, póngase en contacto con su médico lo antes posible para recibir orientación.
¿Tysabri funcionará siempre?
En algunos pacientes que reciben Tysabri, las defensas naturales del organismo pueden impedir que el medicamento funcione correctamente con el tiempo a medida que el organismo produce anticuerpos contra el medicamento. Su médico puede decidir si este medicamento no le está funcionando correctamente a partir de su análisis de sangre e interrumpirá el tratamiento, si es necesario.
Si tiene cualquier otra duda sobre el uso de Tysabri, pregunte a su médico. Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico. En caso de duda, pregunte a su médico.
Subcutáneo se abrevia como SC en la etiqueta de la jeringa.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Informe a su médico o enfermero inmediatamentesi observa alguno de los siguientes síntomas.
Signos de infección del cerebro
- Cambios en la personalidad y en la conducta tales como confusión, delirio o pérdida de conocimiento
- Convulsiones (ataques epilépticos)
- Dolor de cabeza
- Náuseas/vómitos
- Rigidez de cuello
- Sensibilidad extrema a la luz intensa
- Fiebre
- Erupción cutánea (en cualquier parte del cuerpo)
Estos síntomas pueden deberse a una infección del cerebro (encefalitis o LMP) o de la envoltura que lo recubre (meningitis).
Signos de otras infecciones graves
- Fiebre inexplicable
- Diarrea grave
- Falta de aliento
- Mareo prolongado
- Dolor de cabeza
- Pérdida de peso
- Falta de energía
- Alteración de la visión
- Dolor o enrojecimiento de ojo(s)
Signos de reacción alérgica
- Urticaria (erupción con picor)
- Hinchazón de cara, labios o lengua
- Dificultad para respirar
- Dolor o molestias en el pecho
- Aumento o disminución de la tensión arterial (su médico o enfermera lo advertirán si están controlando su tensión arterial)
Es más probable que ocurran durante o poco después de la inyección.
Signos de un posible problema hepático
- Color amarillo de la piel o del blanco de los ojos
- Oscurecimiento poco habitual de la orina
- Prueba de la función hepática anormal
Si experimenta alguno de los efectos adversos descritos anteriormente o si cree que tiene una infección consulte a su médico o enfermero inmediatamente. Muestre su tarjeta de información para el pacientey este prospecto a cualquier médico o enfermero que le trate, no solo a su neurólogo.
Otros efectos adversos
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- Infección urinaria
- Dolor de garganta y congestión o secreción nasal
- Dolor de cabeza
- Mareos
- Sensación de malestar (náuseas)
- Dolor en las articulaciones
- Cansancio
Frecuentes(pueden afectar a 1 de cada 10 personas)
- Anemia (disminución del número de glóbulos rojos que puede hacer que su piel esté pálida y que se sienta sin aliento o falto de energía)
- Alergia (hipersensibilidad)
- Escalofríos
- Urticaria (erupción con picor)
- Vómitos
- Fiebre
- Dificultad para respirar (disnea)
- Enrojecimiento de la cara o el cuerpo (rubor)
- Infecciones por el virus del herpes
- Molestia alrededor del lugar donde le administraron la inyección. Puede presentar dolor, moratones, enrojecimiento, picazón o hinchazón
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- Alergia grave (reacción anafiláctica)
- Leucoencefalopatía multifocal progresiva (LMP)
- Trastorno inflamatorio tras la suspensión del medicamento
- Hinchazón de la cara
- Aumento de la cantidad de glóbulos blancos (eosinofilia)
- Reducción del número de plaquetas
- Formación de moratones con facilidad (púrpura)
Raros(pueden afectar hasta 1 de cada 1000 personas)
- Infección por el virus del herpes en el ojo
- Anemia grave (disminución del número de glóbulos rojos que puede hacer que su piel esté pálida y que se sienta sin aliento o falto de energía)
- Hinchazón intensa debajo de la piel
- Niveles altos de bilirrubina en sangre (hiperbilirrubinemia)que pueden causar síntomas como coloración amarillenta de los ojos o la piel, fiebre y cansancio
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles)
- Infecciones poco frecuentes del cerebro y los ojos
- Daño en el hígado
Informe a su médico lo antes posiblesi cree que padece una infección.
También encontrará esta información en la tarjeta de información para el paciente que le ha entregado su médico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de TYSABRI
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y la caja. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera. (entre 2 ºC y 8 ºC).
No congelar.
Conservar las jeringas en el embalaje exterior para protegerlas de la luz.
Las jeringas precargadas se pueden conservar a temperatura ambiente (hasta 30 °C) durante un tiempo máximo combinado de hasta 24 horas, incluido el tiempo necesario para que alcancen la temperatura ambiente para la administración. Las jeringas se pueden volver a colocar en la nevera y ser utilizadas antes de la fecha de caducidad indicada en la etiqueta y en la caja. La fecha y la hora a la que se saca el envase de la nevera deben anotarse en la caja. Deseche las jeringas si se dejan fuera de la nevera durante más de 24 horas. No utilice fuentes de calor externas, como agua caliente, para calentar las jeringas precargadas.
No utilice este medicamento si observa partículas en el líquido o cambios de color en el líquido.
6. Contenido del envase e información adicional
Composición de Tysabri
El principio activo es natalizumab.
Cada jeringa precargada de 1 ml contiene 150 mg de natalizumab.
Los demás componentes son:
Fosfato monobásico de sodio monohidrato
Fosfato dibásico de sodio heptahidrato
Cloruro de sodio (ver sección 2 “Tysabri contiene sodio”)
Polisorbato 80 (E 433)
Agua para preparaciones inyectables
Aspecto del producto y contenido del envase
Tysabri es un líquido de incoloro a ligeramente amarillo y de ligeramente opalescente a opalescente.
Cada caja contiene dos jeringas.
Tysabri está disponible en envases que contienen 2 jeringas precargadas.
Titular de la autorización de comercializacióny responsable de la fabricación
Biogen Netherlands B.V.
Prins Mauritslaan 13
1171 LP Badhoevedorp
Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Biogen Belgium N.V./S.A. Tél/Tel: +32 2 219 12 18 | Lietuva Biogen Lithuania UAB Tel: +370 5 259 6176 |
| Luxembourg/Luxemburg Biogen Belgium N.V./S.A. Tél/Tel: +352 2 219 12 18 |
Ceská republika Biogen (Czech Republic) s.r.o. Tel: +420 255 706 200 | Magyarország Biogen Hungary Kft. Tel.: +36 (1) 899 9883 |
Danmark Biogen (Denmark) A/S Tlf.: +45 77 41 57 57 | Malta Pharma MT limited Tel: +356 213 37008/9 |
Deutschland Biogen GmbH Tel: +49 (0) 89 99 6170 | Nederland Biogen Netherlands B.V. Tel: +31 20 542 2000 |
Eesti Biogen Estonia OÜ Tel: +372 618 9551 | Norge Biogen Norway AS Tlf: +47 23 40 01 00 |
Ελλáδα Genesis Pharma SA Τηλ: +30 210 8771500 | Österreich Biogen Austria GmbH Tel: +43 1 484 46 13 |
España Biogen Spain SL Tel: +34 91 310 7110 | Polska Biogen Poland Sp. z o.o. Tel.: +48 22 351 51 00 |
France Biogen France SAS Tél: +33 (0)1 41 37 95 95 | Portugal Biogen Portugal Sociedade Farmacêutica Unipessoal, Lda Tel: +351 21 318 8450 |
Hrvatska Biogen Pharma d.o.o. Tel: +358 (0) 1 775 73 22 | România Johnson & Johnson Romania S.R.L. Tel: +40 21 207 18 00 |
Ireland Biogen Idec (Ireland) Ltd. Tel: +353 (0)1 463 7799 | Slovenija Biogen Pharma d.o.o. Tel: +386 1 511 02 90 |
Ísland Icepharma hf Sími: +354 540 8000 | Slovenská republika Biogen Slovakia s.r.o. Tel: +421 2 323 340 08 |
Italia Biogen Italia s.r.l. Tel: +39 02 584 9901 | Suomi/Finland Biogen Finland Oy Puh/Tel: +358 207 401 200 |
Κúπρος Genesis Pharma (Cyprus) Ltd Τηλ: +357 22 76 57 15 | Sverige Biogen Sweden AB Tel: +46 8 594 113 60 |
Latvija Biogen Latvia SIA Tel: +371 68 688 158 |
Fecha de la última revisión de este prospecto:05/2025
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu
--------------------------------------------------------------------------------------------------------------------
INSTRUCCIONES DE USO
Tysabri 150mg
solución inyectable
natalizumab
inyección por vía subcutánea
Dosis completa=Dos jeringas precargadas
Estas «Instrucciones de uso» contienen información sobre cómo inyectar el medicamento utilizando la jeringa precargada de Tysabri.
Lea estas Instrucciones de uso antes de empezar a utilizar la jeringa precargada de Tysabri (denominada «jeringa» en estas instrucciones) y cada vez que obtenga un nuevo envase. Puede haber información nueva.
Esta información no sustituye la consulta con su profesional sanitario sobre su afección médica o su tratamiento.
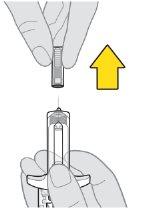
Partes del dispositivo de Tysabri
No retire las alas de sujeción. Las alas de sujeción le permitirán sujetar la jeringa con más firmeza durante el proceso de inyección.
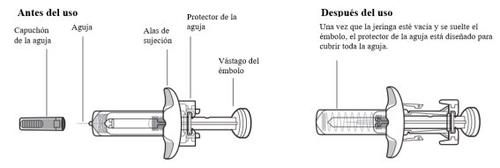
Información importante que debe saber antes de inyectar Tysabri
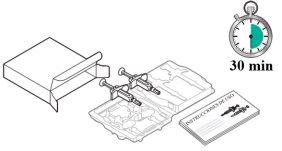
Tysabri se presenta en una jeringa precargada (denominada «jeringa» en estas instrucciones). Cada caja de Tysabri contiene dos jeringas. Debe utilizar ambas jeringas, en un plazo máximo de 30 minutos entre ellas, para obtener su dosis completa.
- En caso de autoadministración o administración por un cuidador, su profesional sanitario debe enseñarle a usted o a su cuidador cómo preparar e inyectar las jeringas antes de utilizarlas por primera vez. Si usted o su cuidador administran las inyecciones y han omitido una dosis o han inyectado solo una jeringa, póngase en contacto con su farmacéutico o el médico que le trata.
- Las jeringas son únicamente para inyección subcutánea (inyectar directamente en la capa grasa debajo de la piel).
- Cada jeringa solo se puede utilizar una vez (un solo uso). No se pueden reutilizar.
- No comparta las jeringas con otras personas, aunque tengan la misma enfermedad que usted. Podría contagiarles una infección o contraer una infección de ellos.
Nota para profesionales sanitarios:
Se debe supervisara los pacientes durante las inyecciones subcutáneas y durante 1hora despuéspara detectar signos y síntomas de reacciones a la inyección, incluida la hipersensibilidad. Después de las seis primeras dosis de Tysabri, independientemente de la vía de administración, se debe supervisar a los pacientes después de la inyección subcutánea según criterio médico.
Conservación deTysabri
- Mantener la jeringa y todos los medicamentos fuera de la vista y del alcance de los niños.
- Conservar las jeringas en la nevera (entre 2 °C y 8 °C).
- En caso necesario, se pueden conservar las jeringas a temperatura ambiente (hasta 30°C) y hasta 24horas en total. Si las jeringas han estado fuera de la nevera durante más de 24horas, no las utilice.
- Conservar las jeringas en la caja original para protegerlas de la luz.
- No congelar las jeringas ni exponerlas a temperaturas superiores a 30 °C.
- Las jeringas se pueden volver a colocar en la nevera y ser utilizadas antes de la fecha de caducidad indicada en la etiqueta y en la caja.
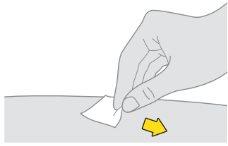
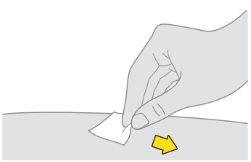
Preparación de la inyección de Tysabri:
|
|
|
|
Noutilice fuentes de calor externas, como agua caliente, para calentar las jeringas. | |
|
|
|
|
Noutilice la jeringa si ha pasado la fecha de caducidad. | |
| |
Noutilice la jeringa si está dañada o agrietada. | |
| |
Noutilice la jeringa si el líquido tiene partículas visibles. Noutilice la jeringa si se ha caído antes de usarla. Informe a su profesional sanitario si tiene alguno de estos problemas con las jeringas | |
Es posible que vea burbujas en el medicamento. Esto es normal. Nota:el aspecto del medicamento puede cambiar después de sacarlo de la nevera. Esto es normal | |
|
|
|
|
Notoque, abanique ni sople sobre la zona limpia. |
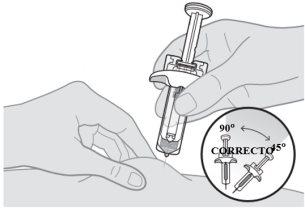
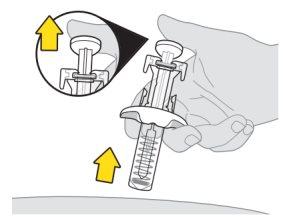
Inyección de la primera jeringa
Nota:es posible que vea una gota de líquido en la punta de la aguja. Esto es normal. |
|
| |
|
|
|
|
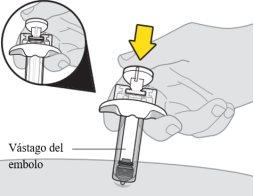
Asegúrese de empujar el vástago del émbolo hasta el fondo para que salga todo el medicamento y encaje el protector de la aguja. | |
|
|
Si el protector de la aguja no se activa para cubrir la aguja, novuelva a colocar el capuchón en la jeringa. Deposítela en el recipiente para objetos punzantes y póngase en contacto con su profesional sanitario para obtener ayuda. | |
|
|
Inyección de la segundajeringa
|
|
|
|
No toque, abanique ni sople sobre la zona limpia. | |
Administre las inyecciones una tras otra sin un retraso significativo. La segunda inyección se debe administrar a más tardar 30 minutos después de la primera. |
|
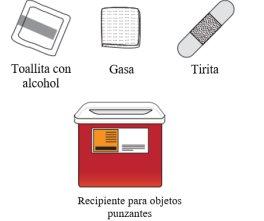
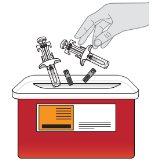
Eliminación de Tysabri
|
|
No tire el recipiente para objetos punzantes ni las jeringas usadas a la basura doméstica. | |
Si no dispone de un recipiente para objetos punzantes, puede solicitar uno a su profesional sanitario o puede utilizar un recipiente doméstico que:
Cuando el recipiente para objetos punzantes esté casi lleno, debe seguir las guías de su comunidad sobre la forma correcta de desecharlo. Puede haber normativas estatales o locales sobre cómo debe desechar las jeringas usadas. No tire el recipiente para objetos punzantes usado a la basura doméstica a menos que las normas de su comunidad lo permitan. No recicle el recipiente para objetos punzocortantes. |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TYSABRI 150 MG SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE PERFUSION, 300 mgPrincipio activo: NatalizumabFabricante: Sandoz GmbhRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 20 mgPrincipio activo: NatalizumabFabricante: Biogen Netherlands B.V.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 120 mg (80 mg/kg) belimumabPrincipio activo: BelimumabFabricante: Glaxosmithkline (Ireland) LimitedRequiere receta
Médicos online para TYSABRI 150 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TYSABRI 150 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes