TYENNE 162 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar TYENNE 162 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Tyenne 162 mg solución inyectable en pluma precargada
tocilizumab
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Además de este prospecto, se le dará una Tarjeta de Información para el paciente, que contiene información importante de seguridad que debe conocer antes de recibir y durante el tratamiento con Tyenne.
Contenido del prospecto
- Qué es Tyenne y para qué se utiliza
- Qué necesita saber antes de empezar a usar Tyenne
- Cómo usar Tyenne
- Posibles efectos adversos
- Conservación de Tyenne
- Contenido del envase e información adicional
1. Qué es Tyenne y para qué se utiliza
Tyenne contiene una sustancia activa llamada tocilizumab, que es una proteína obtenida a partir de células inmunitarias específicas (anticuerpo monoclonal), que bloquea la acción de un tipo de proteína específica (citoquina) llamada interleucina-6. Esta proteína está implicada en procesos inflamatorios del cuerpo, y bloqueándola se puede reducir la inflamación. Tyenne está indicado para tratar:
- adultos con artritis reumatoide activa (AR) de moderada a grave, que es una enfermedad autoinmune, si los tratamientos previos no han funcionado bien.
- adultos con artritis reumatoide (AR) grave, activa y progresiva, que no han sido previamente tratados con metotrexato.
Tyenne ayuda a reducir los síntomas de la AR tales como el dolor y la hinchazón en sus articulaciones y puede también mejorar así su rendimiento en las tareas diarias. Tyenne ha demostrado disminuir la progresión del daño en el cartílago y los huesos de sus articulaciones causados por la enfermedad y mejorar su capacidad para realizar sus actividades diarias.
Tyenne normalmente se utiliza en combinación con otro medicamento para la AR llamado metotrexato. Sin embargo, Tyenne se le puede administrar solo, si su médico determina que el metotrexato no es adecuado.
- adultos con una enfermedad de las arterias llamada arteritis de células gigantes (ACG), causada por la inflamación de las arterias más grandes del cuerpo, especialmente aquellas que suministran sangre a la cabeza y al cuello. Los síntomas pueden incluir dolor de cabeza, fatiga (cansancio) y dolor en la mandíbula. Los efectos pueden incluir derrames cerebrales y ceguera.
Tyenne puede reducir el dolor e hinchazón de las arterias y venas de cabeza, cuello y brazos.
La ACG se trata a menudo con medicamentos llamados esteroides. Por lo general son eficaces, pero pueden tener efectos secundarios si se usan a dosis altas durante mucho tiempo. La reducción de la dosis de esteroides también puede conducir a un brote de ACG. La adición de Tyenne al tratamiento hace que el tiempo de uso de los esteroides pueda ser más corto, mientras que siguen controlando la enfermedad.
- niños y adolescentes, de 12 años de edad y mayores, con artritis idiopática juvenil sistémica (AIJs) activa,una enfermedad inflamatoria que causa dolor e hinchazón en una o más articulaciones, así como fiebre y erupción cutánea.
Tyenne se utiliza para mejorar los síntomas de la AIJs. Se puede administrar en combinación con metotrexato o solo.
- niños y adolescentes, de 12 años de edad en adelante, con artritis idiopática juvenil poliarticular activa (AIJp).Ésta es una enfermedad inflamatoria que causa dolor e hinchazón en una o más articulaciones.
Tyenne se utiliza para mejorar los síntomas de la AIJp. Se puede administrar en combinación con metotrexato o solo.
2. Qué necesita saber antes de empezar a usar Tyenne
No se le administrará Tyenne
- Si es alérgicoal tocilizumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Si tiene una infección activa grave.
Si le sucede algo de esto, consulte con el médico o el enfermero que le administra la perfusión.
Advertencias y precauciones
Consulte a su médico o enfermero antes de empezar a recibir Tyenne.
- Si experimenta reacciones alérgicascomo sensación de opresión torácica, sibilancias, mareos o aturdimiento intenso, hinchazón de los labios o erupción cutánea durante o después de la perfusión, informe a su médico inmediatamente.
- Si tiene cualquier tipo de infección,ya sea de evolución corta o larga, o si contrae infecciones a menudo. Informe inmediatamente a su médicosi se encuentra mal. Tyenne puede reducir la capacidad de su cuerpo para responder a las infecciones y puede hacer que una infección existente empeore o aumente la probabilidad de adquirir una nueva infección.
- Si ha tenido tuberculosis, informe a su médico. Su médico comprobará los signos y síntomas de tuberculosis antes de comenzar el tratamiento con Tyenne. Informe a su médico inmediatamente si los síntomas de tuberculosis (tos persistente, pérdida de peso, malestar general, febrícula), o cualquier otra infección aparecen durante o después del tratamiento.
- Si ha tenido úlcera intestinal o diverticulitis, informe a su médico. Los síntomas incluirían dolor abdominal y cambios inexplicables en los hábitos intestinales con fiebre.
- Si tiene enfermedad hepática, informe a su médico. Antes de usar Tyenne, su médico le realizará un análisis de sangre para medir su función hepática.
- Si algún paciente ha sido vacunado recientemente(adulto o niño) o tiene previsto vacunarse, informe a su médico. Todos los pacientes, especialmente los niños deben estar al día con su calendario de vacunación antes de comenzar el tratamiento con Tyenne, a no ser que se requiera iniciar tratamiento urgente. Determinados tipos de vacunas no deben administrarse mientras reciba Tyenne.
- Si tiene cáncer, avise a su médico. Su médico tendrá que decidir si puede seguir recibiendo tratamiento con Tyenne.
- Si tiene factores de riesgo cardiovascular, tales como aumento de la presión arterial,
valores altos de colesterol, informe a su médico. Estos factores necesitan ser controlados mientras recibe tratamiento con Tyenne.
- Si tiene problemas de riñón de moderados a graves, su médico le vigilará.
- Si tiene dolores de cabeza persistentes.
Su médico le realizará análisis de sangre antes de que reciba Tyenne, y durante su tratamiento, para determinar si tiene un recuento bajo de glóbulos blancos sanguíneos, un recuento bajo de plaquetas o elevación de las enzimas hepáticas.
Niños y adolescentes
No se recomienda el uso de Tyenne en niños menores de 2 años.
Avise a su médico, si el niño tiene antecedentes del síndrome de activación de macrófagos, (activación y proliferación incontrolada de células específicas de la sangre). Su médico decidirá si puede seguir recibiendo Tyenne.
Otros medicamentos y Tyenne
Informe a su médico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento (o si los está tomando su hijo, si él es el paciente). Esto incluye los medicamentos adquiridos sin receta. Tyenne puede afectar a la forma en la que actúan algunos medicamentos, y puede necesitarse un ajuste de dosis. Informe a su médicosi está utilizando medicamentos que contienen cualquiera de estas sustancias activas:
- metilprednisolona, dexametasona, utilizadas para reducir la inflamación,
- simvastatina o atorvastatina, utilizadas para reducir los niveles de colesterol,
- antagonistas de los canales del calcio, como el amlodipino utilizado en el tratamiento del aumento de la presión arterial,
- teofilina, utilizado en el tratamiento del asma,
- warfarina o fenprocumona, utilizados como anticoagulantes,
- fenitoína, utilizado en el tratamiento de las convulsiones,
- ciclosporina, utilizado en los trasplantes de órganos como inmunosupresor,
- benzodiazepinas, como el temazepan utilizado para calmar la ansiedad.
Con respecto a las vacunas, consulte la sección de advertencias anterior.
Debido a que no hay experiencia clínica no se recomienda el uso de Tyenne con otros medicamentos biológicos empleados para tratar la AR, AIJs o AIJp.
Embarazo y lactancia
Tyenne no se debe utilizar durante el embarazo, salvo que sea claramente necesario. Hable con su médico si está embarazada, cree que pudiera estarlo, o tiene previsto quedarse embarazada.
Las mujeres en edad fértil deben utilizar métodos anticonceptivos eficaces durante y hasta 3 meses después de finalizar el tratamiento.
Interrumpa la lactancia si comienza el tratamiento con Tyenne, y consulte con su médico. Antes de reiniciar la lactancia deben haber pasado al menos 3 meses desde su último tratamiento con Tyenne. Se desconoce si Tyenne pasa a la leche materna.
Los datos disponibles hasta el momento no sugieren que este tratamiento tenga ningún efecto sobre la fertilidad.
Conducción y uso de máquinas
Este medicamento puede producir mareos, si usted se siente mareado, no conduzca ni utilice máquinas.
Tyenne contiene sodio
Este medicamento contiene 0,24 mg de sodio (componente principal de la sal de mesa/para cocinar) en cada ml. Esto equivale al 0,012% de la ingesta diaria máxima de sodio recomendada para un adulto. Sin embargo, Tyenne se diluye en solución para perfusión de cloruro de sodio 9 mg/ml (0,9%) o 4,5 mg/ml (0,45%). Esto debe tenerse en cuenta en pacientes con una dieta controlada en sodio.
3. Cómo usar Tyenne
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico, farmacéutico o enfermero. En caso de duda, consulte de nuevo a su médico, farmacéutico o enfermero.
El tratamiento se debe iniciar por un profesional sanitario con experiencia en el diagnóstico y tratamiento de la AR, AIJS, AIJp o ACG.
Adultos con AR o ACG
La dosis recomendadapara todos los adultos con AR (artritis reumatoide) o ACG (arteritis de células gigantes) es de 162 mg (el contenido de una pluma precargada) administrada una vez por semana.
Adolescentes con AIJs (de 12 años de edad en adelante)
La dosis habitual de Tyenne depende del peso del paciente.
- Si el paciente pesa menos de 30 kg: la dosis es de 162 mg (el contenido de 1 pluma precargada), una vez cada 2 semanas.
- Si el paciente pesa 30 kg o más: la dosis es de 162 mg (el contenido de 1 pluma precargada), una vez cada semana.
Adolescentes con AIJp (de 12 años de edad en adelante)
La dosis habitual de Tyenne depende del peso del paciente.
- Si el paciente pesa menos de 30 kg: la dosis es de 162 mg (el contenido de 1 pluma precargada), una vez cada 3 semanas.
- Si el paciente pesa 30 kg o más: la dosis es de 162 mg (el contenido de 1 pluma precargada), una vez cada 2 semanas.
Tyenne se administra mediante inyección debajo de la piel (subcutáneamente). Al inicio, su médico o enfermero puede inyectarle Tyenne. Sin embargo, su médico puede decidir que usted mismo se inyecte Tyenne. En este caso usted recibirá información de cómo autoinyectarse Tyenne. Los padres y cuidadores serán entrenados en cómo inyectar Tyenne a los pacientes que no pueden inyectarse ellos mismos.
Hable con su médico si tiene alguna pregunta sobre como usted o un adolescente al que cuida puede autoadministrarse una inyección. Al final de este prospecto usted encontrará “instrucciones de administración” detalladas.
Si usa más Tyenne del que debe
Como Tyenne se administra en una pluma precargada, es poco probable que se le administre demasiado. Sin embargo, si le preocupa, hable con su médico, farmacéutico o enfermero.
Si un adulto con AR o ACG o un adolescente con AIJs perdió u olvidó una dosis
Es muy importante usar Tyenne exactamente como lo prescribe su médico. Mantenga el registro de su próxima dosis.
- Si usted olvida su dosis semanal dentro de los 7 días, tome su dosis en el próximo día programado.
- Si usted olvida la dosis de cada dos semanas dentro de los 7 días, inyecte una dosis tan pronto como se acuerde y tome su siguiente dosis según su calendario original.
- Si usted se olvida su dosis semanal o cada dos semanas durante más de 7 días o no está seguro cuando inyectarse Tyenne llame a su médico o farmacéutico.
Si un adolescente con AIJp perdió u olvidó una dosis
Es muy importante usar Tyenne exactamente como lo prescribe el médico. Mantenga el registro de la próxima dosis.
- Si olvida una dosis dentro de los 7 días, inyecte una dosis tan pronto como se acuerde y administre la siguiente dosis según su calendario original.
- Si olvida una dosis durante 7 días o más, o no está seguro de cuándo inyectarse Tyenne, llame al médico o al farmacéutico.
Si interrumpe el tratamiento con Tyenne
No debe detener el tratamiento con Tyenne sin consultárselo a su médico previamente.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Los efectos adversos pueden ocurrir hasta al menos 3 meses después de su última dosis de Tyenne.
Posibles efectos adversos graves: consulte con su médico inmediatamente.
Estos son frecuentes: Pueden afectar hasta de 1 de cada 10 personas
Reacciones alérgicasdurante o después de la inyección:
- dificultad para respirar, opresión torácica o aturdimiento,
- erupción cutánea, picor, ronchas, hinchazón de los labios, lengua o cara.
Si experimenta cualquiera de estos síntomas hable con su médico inmediatamente.
Signos de infecciones graves:
- fiebre y escalofríos,
- ampollas en la boca o la piel,
- dolor de estómago.
Signos y síntomas de toxicidad hepática:
Pueden afectar hasta 1 de cada 1 000 personas
- cansancio,
- dolor abdominal,
- ictericia (decoloración amarillenta de piel u ojos).
Si nota alguno de estos síntomas, avise a su médico inmediatamente.
Efectos adversos muy frecuentes:
Pueden afectar a más de 1 de cada 10 personas
- infecciones de las vías respiratorias superiores, con síntomas típicos como tos, congestión nasal, moqueo, dolor de garganta y dolor de cabeza,
- niveles altos de grasa en sangre (colesterol),
- reacciones en el lugar de la inyección.
Efectos adversos frecuentes:
Pueden afectar hasta 1 de cada 10 personas
- infección de pulmón (neumonía),
- herpes (herpes zoster),
- calenturas (herpes simple oral), ampollas,
- infecciones en la piel (celulitis), a veces con fiebre y escalofríos,
- erupción y picor, urticaria,
- reacciones alérgicas (hipersensibilidad),
- infección ocular (conjuntivitis),
- dolor de cabeza, mareos, hipertensión,
- úlceras en la boca, dolor de estómago,
- retención de líquido (edema) en la parte inferior de las piernas, aumento de peso,
- tos, respiración entrecortada,
- recuentos bajos de los glóbulos blancos en análisis de sangre (neutropenia, leucopenia),
- pruebas de función hepática alteradas (elevación de las transaminasas),
- aumento de la bilirrubina medido mediante análisis de sangre,
- niveles bajos de fibrinógeno en sangre (proteína involucrada en la coagulación de la sangre).
Efectos adversos poco frecuentes:
Pueden afectar hasta 1 de cada 100 personas
- diverticulitis (fiebre, náuseas, diarrea, estreñimiento, dolor de estómago),
- zonas hinchadas y rojas en la boca,
- grasas elevadas en la sangre (triglicéridos),
- úlceras estomacales,
- piedras en el riñón,
- hipotiroidismo.
Efectos adversos raros:
Pueden afectar hasta 1 de cada 1 000 personas
- Síndrome de Stevens-Johnson (erupción cutánea, que puede dar lugar a ampollas y descamación grave de la piel),
- Reacciones alérgicas mortales (anafilaxia [mortal]),
- inflamación del hígado (hepatitis), ictericia.
Efectos adversos muy raros:
Pueden afectar hasta 1 de cada 10 000 personas
- recuento bajo de glóbulos blancos, glóbulos rojos y plaquetas en análisis de sangre,
- fallo hepático.
Efectos adversos adicionales en niños y adolescentes con AIJs o AIJp
Los efectos adversos en niños y adolescentes con AIJs o AIJp son en general, similares a los de los adultos. Algunos efectos adversos que se observan con mayor frecuencia en niños y adolescentes son: inflamación de nariz y garganta, dolor de cabeza, náuseas y disminución del recuento de glóbulos blancos.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Tyenne
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase.
Conservar en nevera (entre 2 °C y 8 °C). No congelar.
Mantener los viales en el embalaje exterior para protegerlos de la luz.
6. Contenido del envase e información adicional
Composición de Tyenne
- El principio activo es tocilizumab.
Cada pluma precargada contiene 162 mg de tocilizumab en 0,9 ml.
- Los demás componentes son L-arginina, L-histidina, ácido L-láctico, cloruro de sodio, polisorbato 80, ácido clorhídrico (E507) y/o hidróxido de sodio (E524), agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Tyenne es una solución para inyección. La solución es transparente e incolora a amarillo pálido.
Tyenne es suministrado en plumas precargadas de 0,9 ml que contienen 162 mg de tocilizumab solución para inyección.
Cada envase contiene 1 o 4 plumas precargadas. El envase múltiple contiene 12 (3 envases de 4) plumas precargadas. Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Fresenius Kabi Deutschland GmbH
Else-Kroener-Strasse 1
61352 Bad Homburg v.d.Hoehe
Alemania
Responsable de la fabricación
Fresenius Kabi Austria GmbH
Hafnerstrasse 36
8055 Graz
Austria
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos http://www.ema.europa.eu/.
- Instrucciones de uso
Lea atentamente estas instrucciones de uso antes de utilizar Tyenne pluma precargada.
Lea y siga las instrucciones de uso que vienen con Tyenne pluma precargada antes de empezar a usarla y cada vez que obtenga un recambio. Puede haber información nueva. Esta información no sustituye a la consulta con su médico sobre su enfermedad o tratamiento. Si tiene alguna pregunta sobre el uso de Tyenne pluma precargada, llame a su médico.
Información importante
- Lea el prospecto para el paciente que viene con Tyenne pluma precargada para obtener
información importante que necesita saber antes de usarla.
- Antes de utilizar Tyenne pluma precargada por primera vez, asegúrese de que su médico le muestre la forma correcta de utilizarla.
- Nointente desmontar Tyenne pluma precargada en ningún momento.
- Inyecte siempre Tyenne pluma precargada de la forma que le haya enseñado su médico.
Uso de Tyenne pluma precargada
- La pluma precargada es para autoinyección o administración con la ayuda de un cuidador.
- La pluma precargada es para uso en casa.
- Al inyectarse Tyenne, los niños pueden autoinyectarse si tanto el médico como el cuidador lo consideren apropiado.
- Noreutilice la pluma precargada. La pluma precargada es para una sola dosis (un solo uso).
- Nocomparta su pluma precargada con otra persona. Podría contagiar a otra persona una infección o contraer una infección de ella.
- Noretire el capuchón transparente de la pluma precargada hasta que esté listo para inyectarse.
- Noutilice la pluma precargada si presenta signos de deterioro o si se ha caído.
Conservación de Tyenne pluma precargada
- Conservar Tyenne en la nevera entre 2 ºC y 8 ºC.
- Guarde las plumas precargadas no utilizadas en el envase original para protegerlas de la luz.
- No congelar. Si Tyenne se congela, deséchelo en un contenedor para materiales punzantes.
- Mantenga Tyenne fuera de la exposición al calor o a la luz solar directa.
- Mantenga la pluma precargada fuera del alcance y de la vista de los niños.
- Tyenne puede conservarse a temperatura ambiente entre 20 ºC y 25 ºC en el envase de cartón en el que se presenta durante un máximo de 14 días.
- Tire (deseche) Tyenne en un contenedor de material punzante o resistente a pinchazos si ha estado fuera de la nevera más de 14 días. Una vez almacenado a temperatura ambiente, no vuelva a introducirlo en la nevera.
Viajar con Tyenne plumas precargadas
- Cuando viaje en avión, consulte siempre a su compañía aérea y a su médico sobre la posibilidad de llevar consigo medicamentos inyectables. Lleve siempre Tyenne en su equipaje de mano porque la zona de equipajes puede estar muy fría y podría congelarse.
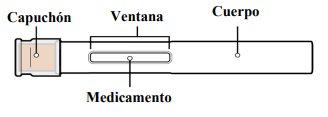
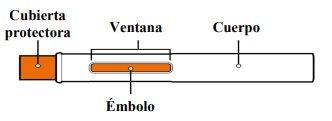
Partes de Tyenne pluma precargada
Antes de usar
Después de usar
PASO 1: Prepare su inyección
1.1. Prepare una superficie plana y limpia, como una mesa o un mostrador, en una zona bien iluminada. | |
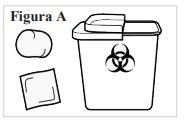
1.2. Usted también necesitará (no incluidos) (ver Figura A):
|
|
1.3. Saque de la nevera la caja que contiene la pluma precargada. Noguarde la pluma precargada fuera de la nevera más de 14 días sin utilizarla. | |
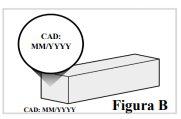
1.4. Compruebe la fecha de caducidad que aparece en la caja para asegurarse de que no ha pasado (ver Figura B). Noutilice la pluma precargada si la fecha de caducidad ha pasado. 1.5. Si es la primera vez que abre la caja, compruebe que la caja no presenta ningún signo de deterioro. |
|
Noutilice la pluma precargada si la caja parece dañadao ha sido abierta. | |
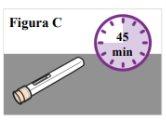
1.6. Abre la caja y extraiga una pluma precargada de un solo uso. Nosujete la pluma precargada por el capuchón. 1.7. Devuelva las plumas precargadas que se queden en la caja a la nevera. 1.8. Deje la pluma precargada a temperatura ambiente sobre la superficie preparada durante 45 minutos antes de usarla para que el medicamento de la pluma precargada alcance la temperatura ambiente (ver Figura C). Nota: Si no lo hace, la inyección podría resultar incómoda y podría tardar más tiempo en inyectarse. Nocaliente de cualquier otra manera, como en un microondas, agua caliente o luz solar directa. |
|
Noretire el capuchón transparente de la pluma precargada hasta que esté listo para inyectarse para evitar lesiones. Mantenga Tyenne fuera del alcance de los niños. |
PASO 2: Compruebe la pluma precargada
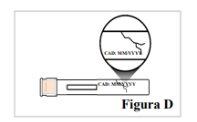
2.1. Compruebe que la pluma precargada no está agrietada ni dañada (ver Figura D). Noutilice la pluma precargada si presenta signos de deterioro o si se ha caído. |
|
2.2. Compruebe la etiqueta de la pluma precargada para asegurarse que:
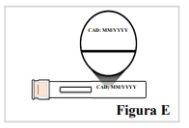
Noutilice la pluma precargada si el nombre en la etiqueta no es Tyenne o la fecha de caducidad ha pasado. |
|
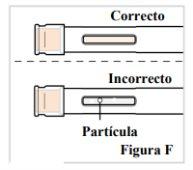
2.3. Observe el medicamento por la ventana. Asegúrese de que sea transparente e incoloro a amarillo pálidoy que no contenga escamas o partículas (ver Figura F). Nota: Es normal que haya burbujas de aire en el medicamento. Nose inyecte si el líquido está turbio, descolorido o tiene grumos o partículas porque puede no ser seguro utilizarlo. |
|
PASO 3: Lávese las manos
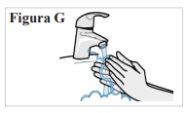
3.1. Lávese las manos con agua y jabón y séqueselas bien con una toalla limpia (ver Figura G). |
|
PASO 4: Elija el sitio de inyección
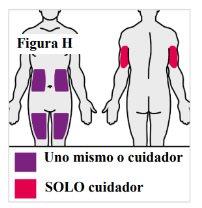
4.1. Si se pone usted mismo la inyección, puede utilizar:
Nota: Elija un lugar diferente para cada inyección para reducir el enrojecimiento, la irritación u otros problemas cutáneos. Noinyecte en piel dolorida (sensible), amoratada, enrojecida, dura, escamosa o con lesiones, lunares, cicatrices, estrías o tatuajes. Noutilice la pluma precargada a través de la ropa. |
|
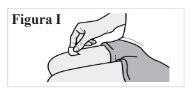
PASO 5: Limpie el lugar de inyección
5.1. Limpie la piel del lugar de la inyección con una toallita con alcohol (ver Figura I). Deje que la piel se seque. Nosople ni toque el lugar después de la limpieza. |
|
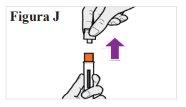
PASO 6: Aplíquese su inyección
6.1. Cuando esté listo para inyectarse, sostenga la pluma precargada en una mano con el capuchón transparente hacia arriba. Con la otra mano, tire firmemente del capuchón transparente hacia fuera sin girarlo (ver Figura J). Nota:Utilice la pluma precargada inmediatamentedespués de retirar el capuchón para evitar la contaminación. Nointente volver a tapar la aguja en ningún momento, ni siquiera al final de la inyección. Notoque la tapa de la aguja (la parte naranja situada en la punta de la pluma precargada) porque podría pincharse accidentalmente. 6.2. Deseche el capuchón transparente. 6.3. Gire la pluma precargada de modo que la cubierta naranja de la aguja apunte hacia abajo. |
|
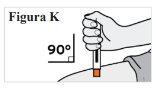
6.4. Coloque la mano sobre la pluma precargada de modo que pueda ver la ventana. 6.5. Coloque la pluma precargada contra su piel en un ángulo de 90 grados (recto) (ver Figura K). Nota: Para asegurarse de que inyecta bajo la piel (en el tejido adiposo), no sostenga la pluma precargada en ángulo. Nota:Noes necesario que pellizque la piel. |
|
Para asegurarse de que inyecta la dosis completa, lea todos los pasos del 6.6 al 6.9 antes de empezar: |
|
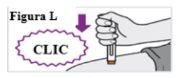
6.6. Con un solo movimiento, empuje la pluma precargada firmemente contra su piel hasta que oiga un primer clic. El émbolo naranja se moverá a través de la ventana durante la inyección (esto significa que la inyección ha comenzado) (ver Figura L). |
|
6.7. ESPERE y mantenga la pluma precargada en su sitio hasta que oiga un segundo clic. Esto puede tardar hasta 10 segundos. Continúe MANTENIENDO (ver Figura M). |
|
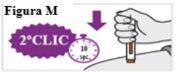
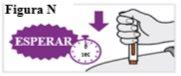
6.8. Espere y cuente lentamente hasta 5 después de oír el segundo clic. Continúe MANTENIENDO la pluma precargada en su sitio para asegurarse de que inyecta una dosis completa (ver Figura N). Nolevante la pluma precargada hasta que esté seguro de que han transcurrido 5 segundos y la inyección se ha completado. |
|
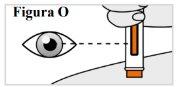
6.9. Mientras sujeta la pluma precargada en su sitio, compruebe la ventana para asegurarse que la varilla naranja del émbolo ha aparecido completamente en la ventana y ha dejado de moverse (ver Figura O), Nota: Si el émbolo naranja no ha bajado del todo o si cree que no ha recibido una inyección completa, llame a su médico. Nointente repetir la inyección con una nueva pluma precargada. |
|
PASO 7: Retire y compruebe la pluma precargada
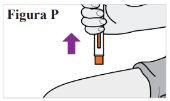
7 7.1. Una vez finalizada la inyección, separe la pluma precargada de la piel (ver Figura P). Nota:La tapa de la aguja se deslizará hacia abajo y cubrirá la aguja. Novuelva a tapar la pluma precargada. |
|
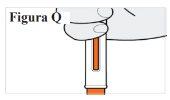
7.2. Compruebe la ventana para asegurarse de que el émbolo naranja ha bajado completamente (ver Figura Q). Nota: Si el émbolo naranja no ha bajado del todo o si cree que no ha recibido una inyección completa, llame a su médico. Nointente repetir la inyección con una nueva pluma precargada. |
|
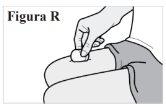
7.3. Si observa sangre en el lugar de la inyección, presione una gasa o una bola de algodón contra la piel hasta que deje de sangrar (ver Figura R). Nofrote el lugar de la inyección. |
|
PASO 8: Deseche su pluma precargada
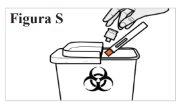
8.1. Deposite la pluma precargada usada en un contenedor para materiales punzantes inmediatamente después de usarla (véase la Figura S). Novuelva a colocar el capuchón transparente en la pluma precargada. Notire (deseche) la pluma precargada a la basura doméstica. Noreutilice la pluma precargada. Si no dispone de un contenedor para materiales punzantes, puede utilizar un recipiente doméstico que:
Cuando el contenedor para eliminar materiales punzantes esté casi lleno, tendrá que seguir las recomendaciones locales para la correcta eliminación del contenedor. Notire (deseche) el contenedor de objetos punzantes usado en la basura doméstica a menos que las normas locales lo permitan. Norecicle el recipiente para materiales punzantes usado. Mantenga siempre el recipiente para objetos punzantes fuera del alcance de los niños. |
|
PASO 9: Anote su inyección
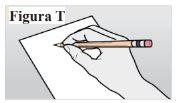
9.1. Anote la fecha y el lugar de la inyección (ver Figura T). Nota:Esto le ayudará a recordar cuándo y dónde debe ponerse la próxima inyección. |
|
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TYENNE 162 MG SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 162 mgPrincipio activo: TocilizumabFabricante: Celltrion Healthcare Hungary Kft.Requiere recetaForma farmacéutica: INYECTABLE, 162 mgPrincipio activo: TocilizumabFabricante: Celltrion Healthcare Hungary Kft.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 20 mg/mlPrincipio activo: TocilizumabFabricante: Celltrion Healthcare Hungary Kft.Requiere receta
Médicos online para TYENNE 162 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TYENNE 162 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes