
TRISEQUENS COMPRIMIDOS RECUBIERTOS

Cómo usar TRISEQUENS COMPRIMIDOS RECUBIERTOS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
PROSPECTO: INFORMACIÓN PARA LA USUARIA
Trisequens comprimidos recubiertos
estradiol/ acetato de noretisterona
Lea todo el prospecto detenidamente antes de empezar a tomar el medicamento, porquecontiene información importante para usted
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado a usted y no debe dárselo a otras personas aunque tengan
los mismos síntomas, ya que puede perjudicarles.
- Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto
adverso no mencionado en este prospecto, informe a su médico o farmacéutico. Ver sección 4.
.
Contenido del prospecto:
- Qué es Trisequens y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Trisequens
- Cómo tomar Trisequens
- Posibles efectos adversos
- Conservación de Trisequens
- Contenido del envase e información adicional
1. Qué es Trisequens y para qué se utiliza
Trisequens es una terapia hormonal sustitutiva (THS) combinada secuencial que se toma cada día sin interrupciones. Trisequens se utiliza en mujeres posmenopáusicas cuyo último período natural se produjo hace 6 meses como mínimo.
Trisequens contiene 2 hormonas, un estrógeno (estradiol) y un progestágeno (acetato de noretisterona). El estradiol en Trisequens es idéntico al estradiol producido por los ovarios de la mujer, y está clasificado como un estrógeno natural. El acetato de noretisterona es un progestágeno sintético que actúa de forma similar a la progesterona, otra importante hormona sexual femenina.
Trisequens se utiliza para:
Aliviar los síntomas que se producen tras la menopausia
Durante la menopausia, la cantidad de estrógenos que produce el cuerpo de la mujer desciende. Esto puede producir síntomas como calor en el rostro, el cuello y el pecho (“sofocos”). Trisequens alivia estos síntomas tras la menopausia. Solo se le prescribirá Trisequens si los síntomas dificultan seriamente su vida diaria.
Prevenir la osteoporosis
Tras la menopausia, algunas mujeres pueden llegar a tener los huesos frágiles (osteoporosis). Deberá comentar con su médico cuáles son las opciones disponibles.
Si aumenta el riesgo de que sufra fracturas debido a la osteoporosis, y no hay otros medicamentos que sean adecuados para usted, puede utilizar Trisequens para prevenir la osteoporosis tras la menopausia.
La experiencia en el tratamiento de mujeres de edad avanzada (más de 65 años) es limitada..
2. Qué necesita saber antes de empezar a tomar Trisequens
Historial médico y revisiones periódicas
El uso de la THS acarrea riesgos que deben tenerse en cuenta antes de decidir comenzar a tomarla o continuar tomándola.
La experiencia en el tratamiento de mujeres con menopausia prematura (debido a un fallo de los ovarios o a una intervención quirúrgica) es limitada. Si usted tiene una menopausia prematura, los riesgos de la THS pueden ser distintos. Consulte a su médico.
Antes de comenzar (o retomar) la THS el médico le preguntará por su historial médico y el de su familia. Podría decidir realizarle una exploración física, que podría incluir una exploración de las mamas o un examen interno, si es necesario.
Una vez que haya comenzado a tomar Trisequens, debería acudir a revisiones periódicas (una vez al año como mínimo). En dichas revisiones podrá comentar con el médico los beneficios y riesgos de continuar el tratamiento con Trisequens.
Acuda a realizarse regularmente exploraciones mamarias, tal como le recomiende su médico.
No tome Trisequens
Si le afecta cualquiera de las siguientes situaciones . Si tiene dudas sobre alguno de los puntos siguientes, pregunte a su médicoantes de tomar Trisequens.
No tome Trisequens
- si tiene, ha tenido o sospecha que pueda tener cáncer de mama
- si tiene, ha tenido o sospecha que pueda tener cáncer de las células que revisten el útero (cáncer de endometrio) u otro cáncer dependiente de estrógenos
- si sufre cualquier hemorragia vaginal de causa desconocida
- si tiene un engrosamiento excesivo de la capa que reviste el útero (hiperplasia endometrial) que no esté siendo tratada
- si tiene o ha tenido alguna vez coágulos sanguíneos en una vena (tromboembolismo venoso), ya sea en las piernas (trombosis venosa profunda) o en los pulmones (embolismo pulmonar)
- si padece algún trastorno de la coagulación (como deficiencia de proteína C, proteína S o antitrombina)
- Si tiene o ha tenido anteriormente un ataque al corazón, un derrame cerebral o angina (angina de pecho) que le causa malestar, presión o dolor en el pecho
- si tiene o ha tenido alguna enfermedad del hígado y los análisis de función hepática todavía no se han normalizado
- si es usted alérgica (hipersensible) al estradiol, el acetato de noretisterona o cualquiera de los demás componentes de Trisequens(detallados en la sección 6 Contenido del envase e información adicional)
- si tiene un raro trastorno sanguíneo denominado “porfiria”, de transmisión hereditaria
Si alguno de estos trastornos se manifiesta por primera vez mientras está tomando Trisequens, suspenda el tratamiento inmediatamente y consulte a su médico lo antes posible.
Advertencias y precauciones
Antes de comenzar el tratamiento, informe a su médico si ha tenido alguna vez cualquiera de los problemas siguientes, ya que podrían volver a presentarse o empeorar durante el tratamiento con Trisequens. Si es así, deberá acudir a su médico con más frecuencia:
- tumores benignos en el útero (fibromas)
- engrosamiento de la capa que reviste el útero (endometriosis) o antecedentes de engrosamiento excesivo de la capa que reviste el útero (hiperplasia endometrial)
- mayorl riesgo de formación de coágulos sanguíneos (ver “Coágulos sanguíneos en una vena (tromboembolismo venoso)”)
- mayorl riesgo de sufrir un cáncer sensible a los estrógenos (como haber tenido una madre, hermana o abuela paterna o materna que hayan padecido un cáncer de mama)
- presión arterial elevada
- un trastorno del hígado, como un tumor hepático benigno
- diabetes
- cálculos biliares
- migraña o dolores de cabeza intensos
- una enfermedad del sistema inmunitario que afecta a varios órganos del cuerpo (lupus eritematoso sistémico o LES)
- epilepsia
- asma
- una enfermedad que afecta a la membrana del tímpano y a la audición (otosclerosis)
- un nivel muy alto de grasa en la sangre (triglicéridos)
- retención de líquidos debido a problemas de corazón o hepáticos
- una condición hereditaria que causa episodios recurrentes de hinchazón grave (angioedema hereditario) o si ha tenido episodios de hinchazón rápida de las manos, la cara, los pies, los labios, los ojos, la lengua, la garganta (obstrucción de las vías respiratorias) o el tracto digestivo (angioedema adquirido).• intolerancia a la lactosa.
Interrumpa el tratamiento con Trisequens e informe inmediatamente al médico
Si nota cualquiera de los siguientes síntomas al tomar la THS:
- cualquiera de los trastornos mencionados en la sección No tome Trisequens.
- coloración amarillenta de la piel o el blanco de los ojos (ictericia). Pueden ser síntomas de una enfermedad del hígado.
- hinchazón de la cara, la lengua y/o la garganta y/o dificultad para tragar o urticaria, junto con dificultad para respirar, que son síntomas indicativos de angioedema.
- un gran aumento de la presión arterial (con síntomas como dolor de cabeza, cansancio, mareos).
- dolor de cabeza de tipo migrañoso, que puede presentarse por primera vez.
- si se queda embarazada.
- si nota síntomas de un coágulo sanguíneo, como:
- inflamación con dolor y enrojecimiento de las piernas
- dolor súbito en el pecho
- dificultad para respirar.
Para más información, ver Coágulos sanguíneos en una vena (tromboembolismo venoso).
Nota:Trisequens no es un anticonceptivo. Si han transcurrido menos de 12 meses desde que tuvo la última menstruación o tiene menos de 50 años, puede que aún necesite utilizar un método anticonceptivo para prevenir el embarazo. Pida consejo a su médico.
THS y cáncer
Engrosamiento excesivo del revestimiento del útero (hiperplasia endometrial) y cáncer del revestimiento del útero (cáncer de endometrio)
La THS solo con estrógenos aumenta el riesgo de un engrosamiento excesivo del revestimiento del útero (hiperplasia endometrial) y cáncer del revestimiento del útero (cáncer de endometrio).
El progestágeno de Trisequens protege frente a este riesgo adicional.
Comparación
En mujeres de 50 a 65 años de edad con útero intacto que no toman THS, se diagnosticarán por término medio 5 casos de cáncer de endometrio por cada 1.000 mujeres.
En mujeres con útero intacto de 50 a 65 años de edad que toman THS solo con estrógenos, se diagnosticarán entre 10 y 60 casos de cáncer de endometrio por cada 1.000 usuarias (es decir, entre 5 y 55 casos adicionales), en función de la dosis y la duración del tratamiento.
Hemorragias inesperadas
Tendrá un sangrado una vez al mes (también llamado metrorragia) mientras esté tomando Trisequens. Pero, si sufre hemorragias inesperadas o pérdidas de sangre (manchados) distintas de los sangrados mensuales, que:
- persiste más allá de los primeros 6 meses
- comienza después de haber tomado Trisequens durante más de 6 meses
- continúa después de haber dejado de tomar Trisequens
debe contactar con su médico lo antes posible.
Cáncer de mama
Los datos existentes muestran que el uso de terapia hormonal sustitutiva (THS) con estrógenos-progestágenos combinados o con sólo estrógenos aumenta el riesgo de cáncer de mama. El riesgo adicional depende del tiempo durante el que use la THS. El riesgo adicional se hace patente después de 3 años de uso. Tras suspender la THS, el riesgo adicional disminuirá con el tiempo, pero el riesgo puede persistir durante 10 años o más si ha usado THS durante más de 5 años.
Comparación
En mujeres de 50 a 54 años de edad que no estén utilizando THS, en una media de 13 a 17 de cada 1000 se diagnosticará cáncer de mama en un período de 5 años.
En mujeres de 50 años que inicien una terapia hormonal sustitutiva con solo estrógenos para 5 años, habrá entre 16 y 17 casos por cada 1000 mujeres usuarias (es decir, entre 0 y 3 casos adicionales).
En mujeres de 50 años que inicien tomando una THS con estrógenos-progestágenos durante 5 años, habrá entre 21 casos por cada 1000 mujeres usuarias (es decir, de 4- 8 casos).
En mujeres de 50 a 59 años que no estén tomando THS, se diagnosticarán un promedio de 27 casos de cáncer de mama por cada 1000 mujeres en un periodo de 10 años.
En mujeres de 50 años que inicien una terapia hormonal sustitutiva solo con estrógenos durante más de 10 años, habrá 34 casos por cada 1000 mujeres usuarias (es decir, 7 casos adicionales).
En mujeres de 50 años que inicien una THS con estrógenos- progestágenos durante 10 años, habrá 48 casos de cada 1000 usuarias (es decir, 21 casos adicionales).
Examine sus mamas regularmente. Acuda al médico si nota cualquier cambio, como:
- hoyuelos en la piel
- cambios en los pezones
- cualquier bulto que pueda ver o notar.
Además, se aconseja participar en programas de exploraciones de las mamas cuando se lo ofrezcan.
En las exploraciones de las mamas, es importante que informe a su enfermera/profesional sanitario que está tomando THS cuando le realice la exploración por rayos X, ya que este medicamento puede incrementar la densidad de las mamas, que puede afectar al resultado de la mamografía. Cuando la densidad de la mama es mayor, puede ser que la mamografía no detecte todos los bultos.
Cáncer de ovario
El cáncer de ovario se produce con menos frecuencia que el cáncer de mama. El uso de THS con estrógenos solos o con combinación de estrógenos-progestágenos se ha asociado con un riesgo ligeramente mayor de cáncer de ovario.
El riesgo de cáncer de ovario varía con la edad. Por ejemplo, en mujeres de entre 50 y 54 años de edad que no siguen THS, se han observado alrededor de 2 casos de cáncer de ovario por cada 2000 mujeres en un periodo de 5 años. En mujeres en tratamiento con THS durante 5 años, se han observado alrededor de 3 casos por cada 2000 pacientes (es decir, alrededor de 1 caso adicional).
Efecto de la THS en el corazón y la circulación
Coágulos sanguíneos en una vena (tromboembolismo venoso)
El riesgo de coágulos sanguíneos en las venases aproximadamente de 1,3 a 3 veces superior en usuarias de THS que en las no usuarias, especialmente durante el primer año.
Los coágulos sanguíneos pueden ser graves, y si uno de ellos llega a los pulmones, puede provocar dolor en el pecho, dificultad para respirar, síncope o incluso la muerte.
La probabilidad de padecer coágulos sanguíneos en las venas será mayor con el aumento de la edad y si interviene uno de los siguientes factores. En caso de que alguna de estas situaciones pueda aplicarse a usted, informe a su médico:
- si no pudiera caminar durante bastante tiempo debido a una operación quirúrgica mayor, lesión o enfermedad (ver también la sección 3, Si debe someterse a cirugía).
- tiene un sobrepeso importante (IMC > 30 kg/m²).
- sufre un problema de coagulación sanguínea que necesita un tratamiento prolongado con medicación para prevenir los coágulos.
- algún familiar cercano ha tenido alguna vez un coágulo sanguíneo en piernas, pulmones u otros órganos.
- padece lupus eritematoso sistémico (LES).
- tiene cáncer.
Para conocer los síntomas que provoca un coágulo sanguíneo, consulte el apartado Interrumpa el tratamiento con Trisequense e informe inmediatamente a su médico.
Comparación
Se calcula que, durante un período de 5 años, una media de 4 a 7 de cada 1.000 mujeres en la cincuentena que no toman THS sufrirán un coágulo sanguíneo en una vena.
En las mujeres en la cincuentena que toman THS con estrógenos-progestágenos durante 5 años, habrá entre 9 y 12 casos por cada 1.000 usuarias (es decir, 5 casos adicionales).
Cardiopatías (infarto de miocardio)
No se ha demostrado que la THS ayude a prevenir el infarto de miocardio.
Las mujeres de más de 60 años que utilizan la THS con estrógenos-progestágenos presentan un riesgo ligeramente superior de desarrollar cardiopatías que las que no toman THS.
Accidente cerebrovascular
El riesgo de accidente cerebrovascular es aproximadamente 1,5 veces superior en las usuarias de THS que en las no usuarias. El número de casos adicionales de accidente cerebrovascular debido al uso de THS aumentará con la edad.
Comparación
Se calcula que, durante un período de 5 años, por término medio 8 de cada 1.000 mujeres en la cincuentena que no toman THS sufrirán un accidente cerebrovascular.
En las mujeres en la cincuentena que están tomando THS, el número de casos será de 11 por cada 1.000 usuarias durante un período de 5 años (es decir, 3 casos adicionales).
Otras condiciones
La THS no previene la pérdida de memoria. Se ha indicado un mayor riesgo de pérdida de memoria en mujeres que comenzaron a usar THS después de los 65 años. Pida consejo a su médico.
Uso de otros medicamentos
Algunos medicamentos pueden interferir con el efecto de Trisequens. Esto puede provocar hemorragias irregulares. Tales medicamentos son los siguientes:
•Medicamentos para la epilepsia(como fenobarbital, fenitoína y carbamazepina)
•Medicamentos para la tuberculosis(como rifampicina y rifabutina)
- Medicamentos para la infección por el VIH (como nevirapina, efavirenz, ritonavir
y nelfinavir)
- Medicamentos para infecciones de hepatitis C (como telaprevir)
•Preparaciones a base de plantas que contengan hierba de San Juan (Hypericum
perforatum).
THS puede afectar el funcionamiento de ciertos medicamentos:
- Medicamento para epilepsia (lamotrigina), ya que puede aumentar la frecuencia de convulsiones
- Medicamentos para el virus de hepatitis C (VHC) (como el régimen combinado ombitasvir/paritaprevir/ritonavir con o sin dasabuvir, así como un régimen con glecaprevir/pibrentasvir) pueden causar aumentos en los resultados de los análisis de sangre por la función hepática (aumento de la enzima hepática ALT) en mujeres que utilizan anticonceptivos hormonales combinados (AHC) que contienen etinilestradiol. Trisequens contiene estradiol en lugar de etinilestradiol. Se desconoce si puede producirse un aumento de la enzima hepática ALT al utilizar Trisequens con este régimen combinado contra el VHC.
Otros medicamentos pueden aumentar los efectos de Trisequens:
- Medicamentos que contienen ketoconazol (un fungicida)
Trisequens puede afectar al tratamiento concomitante con ciclosporina.
Informe a su médico o farmacéuticosi está tomando o ha tomado recientemente cualquier otro medicamento. Esto también se aplica a los medicamentos sin receta, los medicamentos comprados en otro país, los elaborados a vbase de plantas medicinales, las vitaminas y minerales de alta potencia y los complementos alimenticios. Su médico le aconsejará.
Pruebas analíticas
Si necesita un análisis de sangre, informe a su médico o al personal de laboratorio de que está tomando Trisequens, ya que este medicamento puede alterar los resultados de algunos parámetros de laboratorio.
Toma de Trisequens con los alimentos y bebidas
Los comprimidos pueden tomarse con o sin alimentos o bebidas.
Embarazo y lactancia
Embarazo: No debe tomarTrisequens si está embarazada. Trisequens sólo debe utilizarse en mujeres posmenopáusicas. Si se queda embarazada, deje de tomar Trisequens, debe interrumpir el tratamiento y contactar con su médico.
Lactancia:
No debe tomar Trisequens si está en periodo de lactancia.
Conducción y uso de máquinas
Trisequens no tiene efectos conocidos sobre la capacidad para conducir o utilizar máquinas.
Información importante sobre algunos de los ingredientes de Trisequens
Este medicamento contiene lactosa monohidrato. Si padece una intolerancia a ciertos azúcares, consulte con el médico antes de tomar Trisequens.
Trisequens comprimidos contiene sodio. Trisequens comprimidos contiene menos de 1 mmol de sodio (23 mg) per comprimido; esto es, esencialmente “exento de sodio”..
3. Cómo TOMAR TRISEQUENS
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte a su médico o farmacéutico.
Si no está cambiando de otro producto de terapia hormonal sustitutiva, puede comenzar el tratamiento con Trisequens cualquier día que le convenga. Si está cambiando de otro producto de terapia hormonal sustitutiva, pregunte a su médico cuando debe comenzar el tratamiento con Trisequens.
Tome un comprimido una vez al día, aproximadamente a la misma hora cada día.
Cada envase contiene 28 comprimidos
Días 1 a 12 Tome un comprimido de color azulcada día durante 12 días
Días 13 a 22 Tome un comprimido de color blancocada día durante 10 días
Días 23 a 28 Tome un comprimido de color rojocada día durante 6 días.
Tome el comprimido con un vaso de agua.
Cuando haya terminado el envase, comience un envase nuevo para continuar el tratamiento sin interrupción. Normalmente tendrá un sangrado similar a la menstruación (periodo) al comenzar un nuevo envase.
Para más información sobre el uso del envase calendario consulte INSTRUCCIONES DE USO, al final de este prospecto.
Su médico intentará recetarle la dosis efectiva más baja y durante el menor tiempo posible que le proporcione el alivio de los síntomas. Hable con el médico si cree que la dosis es excesiva o insuficiente.
Hable con su médico si no experimenta alivio de los síntomas después de 3 meses de tratamiento. El tratamiento solo debe mantenerse mientras los beneficios superen a los riesgos.
Si toma más Trisequens del que debe
Si ha tomado más Trisequens del que debe, consulte con un médico o farmacéutico. Una sobredosis de Trisequens puede producir náuseas o vómitos.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó tomar Trisequens
Si ha olvidado tomar el comprimido correspondiente a la hora habitual, tómelo dentro de las siguientes 12 horas. Si ya han pasado más de 12 horas, tómelo al día siguiente a la hora habitual. No tome una dosis doble para compensar la dosis olvidada.
El olvido de una dosis puede aumentar la probabilidad de sufrir metrorragia intercurrente y oligometrorragia.
Si interrumpe el tratamiento con Trisequens
Si usted desea interrumpir el tratamiento con Trisequens, hable en primer lugar con su médico, quien le explicará los efectos de interrumpir el tratamiento y comentará con usted otras posibilidades.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
Si se debe someter a cirugía
Si se va a someter a una intervención quirúrgica, informe al cirujano de que está tomando Trisequens. Puede que tenga que suspender la toma de Trisequens de 4 a 6 semanas antes de la operación para reducir el riesgo de coágulos sanguíneos (ver la sección 2, Coágulos sanguíneos en una vena (tromboembolismo venoso). Pregunte a su médico cuándo puede comenzar a tomar Trisequens de nuevo.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los siguientes trastornos han sido comunicados con más frecuencia en las mujeres que utilizan la THS respecto a las que no la utilizan:
- cáncer de mama
- crecimiento anómalo o cáncer del revestimiento del útero (hiperplasia endometrial o cáncer de endometrio)
- cáncer de ovario
- coágulos sanguíneos en las venas de las piernas o los pulmones (tromboembolismo venoso)
- cardiopatía
- accidente cerebrovascular
- probable pérdida de memoria si se empieza la THS con más de 65 años de edad.
Para obtener más información sobre estos efectos adversos, ver el sección 2, Qué necesita saber antes de empezar a tomar Trisequens.
Hipersensibilidad/alergia(efecto adverso poco frecuente – afecta de 1 a10 usuarias de cada 1.000)
Aunque es un efecto poco frecuente, puede producirse hipersensibilidad/alergia. Los signos de hipersensibilidad/alergia pueden incluir uno o más de los síntomas siguientes: urticaria, picor, hinchazón, dificultad respiratoria, disminución de la presión arterial (palidez y enfriamiento de la piel, aumento del ritmo cardíaco), sensación de mareo y sudores que pueden ser signos de una reacción o choque anafilácticos. Si se produce uno de los síntomas mencionados, deje de tomar Trisequens y solicite inmediatamente ayuda médica.
Efectos adversos muy frecuentes
- Dolor o sensibilidad en las mamas
- Periodos irregulares o sangrado excesivo durante los periodos.
Efectos adversos frecuentes
- Cefalea
- Aumento de peso por retención de líquidos
- Inflamación vaginal
- Infección vaginal por hongos
- Migraña, de nueva aparición o empeoramiento de la existente
- Depresión, de nueva aparición o empeoramiento de la existente
- Náuseas
- Dolor, hinchazón o malestar abdominales
- Aumento de tamaño o hinchazón de las mamas (edema mamario)
- Dolor de espalda
- Calambres en las piernas
- Empeoramiento, aparición o reaparición de fibroma uterino (tumor benigno)
- Hinchazón de brazos y piernas (edema periférico)
- Aumento de peso.
Efectos adversos poco frecuentes
- Flatulencia o meteorismo
- Acné
- Caída del cabello (alopecia)
- Crecimiento anómalo (patrón masculino) del vello
- Picor o habones (urticaria)
- Inflamación de una vena (tromboflebitis superficial)
- Ineficacia farmacológica
- Reacción alérgica
- Hiperplasia endometrial (engrosamiento excesivo de la capa que reviste el útero)
- Periodos dolorosos
- Nerviosismo
Efectos adversos raros
- Embolia pulmonar (coágulo de sangre) (ver Coágulos sanguíneos en una vena en la sección 2 Qué necesita saber antes de empezar a tomar Trisequens)
- Inflamación profunda de una vena asociada a trombosis (coágulo de sangre).
Efectos adversos muy raros
- Cáncer del revestimiento del útero (cáncer de endometrio)
- Aumento de la presión arterial o empeoramiento de la hipertensión existente
- Enfermedad de la vesícula biliar, cálculos biliares que pueden ser de nueva aparición, reaparición o empeoramiento de los ya existentes
- Excesiva secreción de sebo, erupción de la piel
- Edema agudo o recurrente (edema angioneurótico)
- Insomnio, mareos, ansiedad
- Cambios en el deseo sexual
- Alteraciones de la visión
- Pérdida de peso
- Vómitos
- Acidez gástrica
- Picores vaginales y genitales
- Infarto de miocardio y accidente vascular cerebral.
La frecuencia de los efectos adversos posibles, que se enumeran arriba, se define de la siguiente manera:
Muy frecuentes (afectan a más de 1 usuaria de cada 10)
Frecuentes (afectan de 1 a 10 usuarias de cada 100)
Poco frecuentes (afectan de 1 a 10 usuarias de cada 1.000)
Raros (afectan de 1 a 10 usuarias de cada 10.000)
Muy raros (afectan a menos de 1 usuaria de cada 10.000)
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles).
Otros efectos adversos de la THS combinada
Se han comunicado las siguientes reacciones adversas con otras THS:
- diversos trastornos cutáneos:
- pigmentación de la piel, especialmente en la cara y el cuello, conocida como “paño del embarazo” (cloasma)
- nódulos cutáneos rojos y dolorosos (eritema nodular)
- erupción con úlceras o enrojecimientos en forma de diana (eritema polimorfo).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano http://www.notificaRAM.es.
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento
5. Conservación de TRISEQUENS®
Mantener este medicamento fuera de la vista y del alcance de los niños.
No use este medicamento después de la fecha de caducidad que aparece en el envase después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Conservar por debajo de 25ºC. No refrigerar.
Conservar el envase en el embalaje exterior para protegerlo de la luz.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGREde la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.”
6. Contenido del envase e información adicional
Qué contiene Trisequens
- Los principios activos son estradiol y acetato de noretisterona.
Los comprimidos recubiertos azules contienen: estradiol 2 mg (como estradiol hemihidrato)
Los comprimidos blancos contienen: estradiol 2 mg (como estradiol hemihidrato) y acetato de noretisterona 1 mg
Los comprimidos rojos contienen: estradiol 1 mg (como estradiol hemihidrato)
- Los otros ingredientes son: lactosa monohidrato, almidón de maíz, hidroxipropilcelulosa, talco y estearato de magnesio
- El recubrimiento de los comprimidos azules contiene: hipromelosa, talco, dióxido de titanio (E171), carmín índigo (E132) y macrogol 400.
El recubrimiento de los comprimidos blancos contiene: hipromelosa, triacetina y talco
El recubrimiento de los comprimidos rojos contiene: hipromelosa, talco, dióxido de titanio (E171), óxido de hierro rojo (E172) y propilenglicol.
Aspecto del producto y contenido del envase
Los comprimidos recubiertos con película son redondos con un diámetro de 6 mm. Los comprimidos azules están grabados con NOVO 280. Los comprimidos blancos están grabados con NOVO 281. Los comprimidos rojos están grabados con NOVO 282.
Cada envase de 28 comprimidos contiene 12 comprimidos azules, 10 comprimidos blancos y 6 comprimidos rojos.
Presentaciones:
- 28 comprimidos recubiertos.
- 3 x 28 comprimidos recubiertos.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
ISDIN, S.A.
Provençals, 33
08019 Barcelona
España
Responsable de la fabricación
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsvaerd
Dinamarca
Fecha de la última revisión de este prospecto: diciembre 2023
Otras fuentes de información
“La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
INSTRUCCIONESDE USO
Cómo utilizar el envase disco-calendario
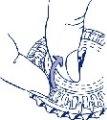
- Colocar el indicador del día
Girar el disco interior y fijar el día de la semana frente a la apertura cerrada con una lengüeta de plástico.

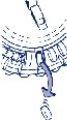
- Cómo extraer el primer comprimido
Romper la lengüeta de plástico y extraer el primer comprimido.

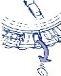
- Mover eldialcada día
Simplemente, girar 1 espacio el disco transparente en la dirección de las agujas del reloj, según indica la flecha. Extraer el siguiente comprimido. Recuerde tomar solo 1 comprimido al
día
El disco transparente solamente se puede girar una vez que se ha extraído el comprimido correspondiente
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TRISEQUENS COMPRIMIDOS RECUBIERTOSForma farmacéutica: COMPRIMIDO, 1mg estradiol, 1mg estradiol/1mg noretisteronaPrincipio activo: norethisterone and estrogenFabricante: Isdin S.A.Requiere recetaForma farmacéutica: COMPRIMIDO, 1 mg/10 mgPrincipio activo: dydrogesterone and estrogenFabricante: Theramex Ireland LimitedRequiere recetaForma farmacéutica: COMPRIMIDO, 2 mg/10mgPrincipio activo: dydrogesterone and estrogenFabricante: Theramex Ireland LimitedRequiere receta
Médicos online para TRISEQUENS COMPRIMIDOS RECUBIERTOS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TRISEQUENS COMPRIMIDOS RECUBIERTOS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






