
ТИССИЛ Раствор для тканевого клея

Спросите врача о рецепте на ТИССИЛ Раствор для тканевого клея

Инструкция по применению ТИССИЛ Раствор для тканевого клея
Введение
Прошу: информация для пользователя
ТИССЕЕЛ Решения для тканевого адгезива
Человеческий фибриноген, человеческая тромбина, синтетическая апротинина, дигидрат хлорида кальция
Прочитайте весь листок внимательно перед началом использования этого лекарства, поскольку он содержит важную информацию для вас.
- Сохраните этот листок, поскольку вам может потребоваться прочитать его снова.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом или фармацевтом.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой, даже если это побочные эффекты, которые не указаны в этом листке. См. раздел 4.
Содержание листка
- Что такое ТИССЕЕЛ и для чего он используется
- Что вам нужно знать перед началом использования ТИССЕЕЛ
- Как использовать ТИССЕЕЛ
- Возможные побочные эффекты
- Хранение ТИССЕЕЛ
- Содержание упаковки и дополнительная информация
1. Что такое ТИССЕЕЛ и для чего он используется
Что это такоеТИССЕЕЛ
ТИССЕЕЛ - это двусоставной тканевой адгезив, состоящий из двух растворов: раствора белка-связующего и раствора тромбина. ТИССЕЕЛ содержит фибриноген и тромбину. Это два важных белка крови, необходимых для свертывания крови. Когда эти белки смешиваются во время применения, они образуют сгусток в месте применения.
Сгусток, образованный ТИССЕЕЛ, очень похож на естественный сгусток. Он разрушается таким же образом, как и естественный сгусток (собственный организм), и не оставляет остатков. В него добавляется синтетический белок (апротинина синтетическая) для увеличения продолжительности сгустка и предотвращения его преждевременного разрушения.
Для чего используетсяТИССЕЕЛ
ТИССЕЕЛ используется как дополнительное лечение, когда традиционные хирургические методы кажутся недостаточными:
- для улучшения гемостаза
- в качестве тканевого герметика для улучшения заживления ран или герметизации швов при сосудистой хирургии и в желудочно-кишечном тракте
- для прикрепления тканей, например, для прикрепления кожных трансплантатов.
ТИССЕЕЛ также эффективен у пациентов, получающих лечение антикоагулянтом гепарином.
2. Что вам нужно знать перед началом использования ТИССЕЕЛ
Не используйте ТИССЕЕЛ:
- если вы аллергичны к активным веществам или любому другому компоненту этого лекарства (перечисленному в разделе 6).
- при сильном артериальном или венозном кровотечении. Одноразовое применение ТИССЕЕЛ не показано в этой ситуации
- ТИССЕЕЛ не должен вводиться в кровеносные сосуды (вены или артерии). Поскольку ТИССЕЕЛ образует сгусток в месте введения, введение в кровеносный сосуд может привести к образованию сгустков крови. Если эти сгустки попадают в кровоток, они могут вызвать потенциально смертельные осложнения
- ТИССЕЕЛ не показан для замены кожных швов, наложенных для закрытия хирургической раны.
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом, фармацевтом или медсестрой перед началом использования ТИССЕЕЛ.
Будьте особенно осторожны с ТИССЕЕЛ, поскольку могут возникнуть аллергические реакции.
Среди первых симптомов аллергической реакции могут быть:
- временное покраснение кожи
- зуд
- крапивница
- тошнота, рвота
- общее недомогание
- озноб
- давление в груди
- отек губ и языка
- затруднение дыхания/одышка
- падение артериального давления
- увеличение или уменьшение частоты пульса
Если появляются эти симптомы, применение должно быть немедленно прекращено. Тяжелые симптомы требуют срочного лечения.
- ТИССЕЕЛ содержит синтетический белок, известный как апротинина. Хотя этот белок применяется только в небольших количествах и только на поверхности раны, существует риск тяжелой аллергической реакции. Риск, кажется, увеличивается у пациентов, которые ранее получали ТИССЕЕЛ или апротинину, даже если это было хорошо перенесено во время предыдущего применения. Поэтому любое использование апротинина или продуктов, содержащих апротинину, должно быть включено в вашу медицинскую историю. Поскольку апротинина синтетическая структурно идентична апротинине бычьей, использование ТИССЕЕЛ у пациентов с аллергией на белки бычьей следует тщательно оценить
- могут возникнуть потенциально смертельные осложнения, если сгустки крови попадают в кровоток из-за случайного введения в кровеносный сосуд.
- Внутривенная инфузия может увеличить вероятность и тяжесть острых гиперчувствительных реакций у восприимчивых пациентов. В частности, во время коронарного шунтирования врач должен быть особенно осторожен, чтобы не вводить ТИССЕЕЛ в кровеносный сосуд. Также важно избегать введения в слизистую носа, поскольку могут образовываться сгустки крови в области офтальмической артерии
- при введении в ткань существует риск местного повреждения ткани
- для предотвращения герметизации тканей в нежелательных зонах. Поэтому перед применением следует позаботиться о покрытии всех частей тела, которые не являются зоной, подлежащей лечению
- образование слишком толстого фибринового сгустка может негативно повлиять на эффективность продукта и процесс заживления раны. Поэтому ТИССЕЕЛ следует наносить тонким слоем.
Следует быть осторожным при применении тканевого адгезива фибрины с использованием сжатого газа.
Были зарегистрированы очень редкие случаи газовой эмболии (воздух или газ) (введение воздуха в кровоток, которое может быть тяжелым или угрожать жизни) в результате использования распылительных устройств с регуляторами давления для применения тканевых адгезивов фибрины. Эти случаи, кажется, связаны с использованием распылительного устройства при давлении выше рекомендуемого и/или на расстоянии очень близко к поверхности ткани. Риск, кажется, выше, когда тканевые адгезивы фибрины распыляются с воздухом по сравнению с CO2, и, следовательно, не могут быть исключены с ТИССЕЕЛ, когда он распыляется во время хирургического вмешательства на открытой ране.
Распылительные устройства и наконечник для применения включают инструкции по использованию, которые рекомендуют интервалы давления и расстояние, на котором следует распылять от поверхности ткани.
ТИССЕЕЛ следует применять точно так, как указано в инструкциях, и только с помощью рекомендуемых для этого продукта устройств.
Всегда, когда ТИССЕЕЛ распыляется, следует контролировать изменения артериального давления, пульса, насыщения кислородом иуровня CO2в конце выдоха, чтобы обнаружить возможную газовую эмболию.
Когда применяются лекарства, полученные из плазмы или человеческой крови, необходимо принять определенные меры, чтобы предотвратить передачу инфекций пациентам. Такие меры включают:
- тщательный отбор доноров, чтобы исключить тех, кто находится в группе риска быть носителями инфекционных заболеваний
- анализ маркеров конкретных инфекций/вирусов в индивидуальных донорских материалах и в плазменных смесях
- включение этапов в процессе производства, которые могут удалить/инактивировать вирусы.
Несмотря на эти меры, когда применяются лекарства, полученные из человеческой крови или плазмы, возможность передачи инфекции не может быть полностью исключена. Это также относится к возникающим вирусам или вирусам неизвестной природы или другим типам инфекций.
Эти меры, принятые для исключения вирусов, такие как ВИЧ, вирус гепатита Б и вирус гепатита С, и для вируса гепатита А, могут иметь ограниченную ценность против невирусных агентов, таких как парвовирус В19. Инфекция парвовирусом В19 может быть тяжелой для беременной женщины (инфекция плода) и для людей с ослабленной иммунной системой или для пациентов с некоторыми формами анемии (например, серповидно-клеточной анемии или гемолитической анемии).
Ваш врач может порекомендовать вам рассмотреть возможность вакцинации против гепатита А и В, если вам регулярно или повторно вводят фибриновые адгезивы, полученные из человеческой плазмы.
Рекомендуется вести учет каждого введения дозы ТИССЕЕЛ, чтобы поддерживать запись использованных партий.
Другие лекарстваи ТИССЕЕЛ
Сообщите вашему врачу или фармацевту, если вы используете, недавно использовали или можете использовать любое другое лекарство, включая те, которые можно купить без рецепта.
Не известны взаимодействия с другими лекарствами.
Как и с подобными продуктами или растворами тромбина, продукт может быть испорчен, если он вступает в контакт с растворами, содержащими алкоголь, йод или тяжелые металлы (например, антисептические растворы). Следует быть осторожным, чтобы исключить эти вещества как можно больше перед применением продукта.
Для информации о препаратах, содержащих окисленную целлюлозу, см. Инструкции по обращению и приготовлению.
Использование ТИССЕЕЛ с продуктами питания и напитками
Спросите вашего врача. Ваш врач решит, можете ли вы есть или пить перед применением ТИССЕЕЛ.
Беременность, лактация и фертильность
Если вы беременны или кормите грудью, думаете, что можете быть беременной или планируете стать беременной, проконсультируйтесь с вашим врачом или фармацевтом перед использованием этого лекарства. Ваш врач решит, можете ли вы использовать ТИССЕЕЛ во время беременности или лактации.
Вождение и использование машин
ТИССЕЕЛ не влияет на вашу способность водить или использовать машины.
ТИССЕЕЛ содержит полисорбат 80
Полисорбат 80 может вызывать местные раздражения кожи, такие как контактный дерматит.
3. Как использовать ТИССЕЕЛ
Использование ТИССЕЕЛ ограничено опытными хирургами, которые были должным образом обучены использованию ТИССЕЕЛ.
Перед применением ТИССЕЕЛ необходимо высушить поверхность раны, используя стандартную технику (например, периодическое применение компрессов, тампонов, использование аспирационных устройств).
Не следует использовать сжатый воздух или газ для сушки области.
ТИССЕЕЛ следует распылять только на видимые зоны применения.
Всегда, когда ТИССЕЕЛ распыляется с помощью распылительного устройства, убедитесь, что вы используете давление и расстояние до ткани, которые находятся в рекомендуемом производителем диапазоне следующим образом:
Давление, расстояние и рекомендуемые устройства для распыления ТИССЕЕЛ | |||||
Хирургическая процедура | Распылительное устройство, которое следует использовать | Наконечники для применения, которые следует использовать | Регулятор давления, который следует использовать | Рекомендуемое расстояние до ткани-мишени | Рекомендуемое давление распыления |
Открытая рана | Распылительное устройство ТИССЕЕЛ/Артисс | н.а. | ИзиСпрей | 10-15 см | 1,5-2,0 бара (21,5-28,5 psi). |
Распылительное устройство ТИССЕЕЛ/Артисс, упаковка 10 | н.а. | ИзиСпрей | |||
Лапароскопические или минимально инвазивные процедуры | н.а. | Наконечник Дуплоспрей МИС 20 см | Регулятор Дуплоспрей МИС 1,5 бара | 2-5 см | 1,2-1,5 бара (18-22 psi) |
Наконечник Дуплоспрей МИС 30 см | |||||
Наконечник Дуплоспрей МИС 40 см | |||||
Эндоскопический набор для распыления 360 со снэп-локом | |||||
Эндоскопический набор для распыления 360 с анкерным креплением | |||||
Заменяемый наконечник |
Всегда, когда ТИССЕЕЛ распыляется, и поскольку существует возможность газовой эмболии (воздух или газ), следует контролировать изменения артериального давления, пульса, насыщения кислородом иуровня CO2в конце выдоха (см. раздел 2).
Доза, которая должна быть введена, всегда зависит от ваших индивидуальных потребностей.
Доза зависит от ряда факторов, таких как тип хирургического вмешательства, размер пораженной поверхности, метод применения и количество применений. Ваш врач решит необходимое количество и применит достаточное для образования тонкого слоя на поражении. Если количество кажется недостаточным, применение может быть повторено.
При применении ТИССЕЕЛ свертывание происходит быстро. Следует избегать нанесения нового слоя на уже существующий слой ТИССЕЕЛ, поскольку новый слой не прилипнет к существующему.
Следует избегать отдельного применения компонента белка-связующего и компонента тромбина.
В клинических испытаниях вводились отдельные дозы 4-20 мл. Возможно, что потребуются более крупные объемы в некоторых процедурах (например, при повреждении печени или герметизации обширных ожогов).
Как ориентировочное руководство для герметизации поверхностей, 1 упаковка ТИССЕЕЛ 2 мл (1 мл раствора белка-связующего плюс 1 мл раствора тромбина) будет достаточна, как минимум, для поверхности 10 см2.
При применении ТИССЕЕЛ с помощью распылительного устройства одно и то же количество будет достаточным для покрытия значительно более крупных площадей.
Для предотвращения чрезмерного образования грануляционной ткани и обеспечения постепенного разрушения тканевого адгезива фибрины следует наносить только тонкий слой ТИССЕЕЛ.
Для обеспечения адекватного смешивания компонента белка-связующего и компонента тромбина следует выпустить и выбросить первые капли продукта из наконечника для применения непосредственно перед его использованием.
Если вы используете больше ТИССЕЕЛ, чем должно быть
ТИССЕЕЛ вводится только во время хирургических вмешательств. Врач определяет необходимое количество. Не известны случаи передозировки.
Если у вас есть какие-либо другие вопросы об использовании этого лекарства, спросите вашего врача или фармацевта.
Дети
Не установлена безопасность и эффективность продукта у детей.
4. Возможные побочные эффекты
Как и все лекарства, это лекарство может вызывать побочные эффекты, хотя не все люди их испытывают. Если вы считаете, что какой-либо из побочных эффектов, которые вы испытываете, является серьезным или если вы заметили любой побочный эффект, не упомянутый в этом листке-вкладыше, сообщите об этом вашему врачу или фармацевту.
У пациентов, леченных с помощью тканевого клея из фибрина, могут возникать реакции гиперчувствительности или аллергические реакции. Хотя они редки, могут быть тяжелыми.
Первые симптомы аллергической реакции могут включать:
- временное покраснение кожи ("приливы")
- зуд
- крапивница
- тошнота, рвота
- головная боль
- онемение
- тревога
- жжение и зуд в месте применения
- ползание
- озноб
- давление в груди
- отек губ, языка, горла (что может привести к затруднению дыхания и/или глотания)
- затруднение дыхания
- низкое кровяное давление
- увеличение или уменьшение частоты пульса
- потеря сознания из-за падения кровяного давления
В отдельных случаях эти реакции могут прогрессировать до тяжелых аллергических реакций (анафилаксии). Эти реакции могут возникать особенно если препарат применяется повторно или если он вводится пациентам, которые ранее показали гиперчувствительность к апротинине или любому другому компоненту продукта.
Даже если повторное лечение ТИССЕЛ было хорошо перенесено, последующая администрация ТИССЕЛ или перфузия апротинина может привести к тяжелым аллергическим реакциям (анафилактическим).
Медицинский персонал, который будет лечить вас, будет осведомлен о риске такого рода реакции и прекратит применение ТИССЕЛ сразу же после появления первых симптомов гиперчувствительности. В случае тяжелых симптомов может быть необходимо принять меры экстренного реагирования.
Введение ТИССЕЛ в мягкие ткани может повредить их местно.
Введение ТИССЕЛ в кровеносные сосуды (вены или артерии) может привести к образованию тромбов (тромбозу).
Поскольку ТИССЕЛ производится из плазмы, полученной из донорской крови, риск инфекций не может быть полностью исключен. Однако производители принимают многочисленные меры для снижения этого риска (см. раздел 2).
Редко могут возникать антитела к компонентам тканевого клея из фибрина.
Следующие побочные эффекты были зарегистрированы во время лечения ТИССЕЛ:
Побочные эффекты были оценены с использованием следующих категорий частоты:
Очень часто: могут возникать у более чем 1 из 10 человек
Часто: могут возникать у до 1 из 10 человек
Не часто: могут возникать у до 1 из 100 человек
Редко: могут возникать у до 1 из 1000 человек
Очень редко: могут возникать у до 1 из 10 000 человек
Частота неизвестна: не может быть оценена на основе доступных данных.
Общие области | Побочный эффект | Частота |
Инфекции и паразитарные заболевания | Постхирургическая инфекция раны | Часто |
Расстройства крови и лимфатической системы | Увеличение продуктов деградации фибрина | Не часто |
Расстройства иммунной системы | Реакции гиперчувствительности | Не часто |
Аллергические реакции (анафилактические) | Не часто | |
Анафилактический шок | Не часто | |
Чувство ползания, жжения или онемения кожи | Не часто | |
Давление в груди | Не часто | |
Затруднение дыхания | Не часто | |
Зуд | Не часто | |
Покраснение кожи | Не часто | |
Расстройства нервной системы | Сенсорные расстройства | Часто |
Расстройства сердца | Увеличение или уменьшение частоты пульса | Не часто |
Расстройства сосудов | Тромбоз вен | Часто |
Падение кровяного давления | Редко | |
Синяки | Не часто | |
Воздушные пузырьки в сосудистой системе* | Частота неизвестна | |
Тромб в кровеносных сосудах | Не часто | |
Закупорка артерии в мозге | Не часто | |
Расстройства дыхания и грудной клетки | Диспноэ | Не часто |
Расстройства желудочно-кишечного тракта | Тошнота | Не часто |
Закупорка кишечника | Не часто | |
Расстройства кожи и подкожной клетчатки | Кожная сыпь | Часто |
Крапивница | Не часто | |
Нарушение заживления | Не часто | |
Расстройства мышечно-скелетной системы и соединительной ткани | Боль в конечностях | Часто |
Общие расстройства и изменения в месте введения | Боль | Часто |
Увеличение температуры тела | Часто | |
Покраснение кожи | Не часто | |
Отек, вызванный накоплением жидкости в тканях (отек) | Не часто | |
Травматические повреждения, отравления и осложнения терапевтических процедур | Боль, вызванная процедурой | Не часто |
Накопление лимфы или других прозрачных жидкостей тела near зоны операции (серома) | Очень часто | |
Быстрое образование отека кожи, подкожной клетчатки, слизистой и подслизистой (ангиоэдем) | Не часто |
*Были зарегистрированы случаи введения воздушных пузырьков или газа в сосудистую систему при применении клеев фибрина с помощью распылительного оборудования, использующего газ или воздух под давлением; считается, что причиной этого эффекта является неправильное использование распылительного оборудования (например, при давлении выше рекомендуемого и на расстоянии, близком к поверхности ткани).
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, проконсультируйтесь с вашим врачом или медсестрой, даже если это возможные побочные эффекты, не указанные в этом листке-вкладыше. Вы также можете сообщить об этом直接 через систему фармакологического надзора за лекарствами для человека: www.notificaram.es.
Сообщая о побочных эффектах, вы можете способствовать предоставлению более полной информации о безопасности этого лекарства.
5. Хранение ТИССЕЛ
Храните это лекарство в недоступном для детей месте.
Не используйте это лекарство после даты истечения срока годности, указанной на упаковке после "CAD".
Храните и перевозите при температуре ниже -20 °C без перерыва до применения.
Храните шприц в наружной упаковке, чтобы защитить его от света.
Хранение после размораживания:
Неразмороженный продукт при комнатной температуре можно хранить до 72 часов при контролируемой комнатной температуре (не выше 25 °C).
Как только разморожено, раствор не должен быть повторно заморожен или охлажден!
Лекарства не должны выбрасываться в канализацию или мусор. Спросите у вашего фармацевта, как избавиться от упаковок от лекарств, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержимое упаковки и дополнительная информация
Состав ТИССЕЛ
ТИССЕЛ содержит два компонента:
Компонент 1: Раствор белка-клея
Активные вещества в 1 мл раствора белка-клея: человеческий фибриноген, 91 мг/мл; синтетическая апротинина, 3000 УИК/мл.
Другие компоненты: человеческий альбумин, L-гистидин, ниацинамид, полисорбат 80 (Твин 80), цитрат натрия дигидрат, вода для инъекций.
Компонент 2: Раствор тромбина
Активные вещества в 1 мл раствора тромбина: человеческая тромбина 500 ЕД/мл; хлорид кальция дигидрат 40 мкмоль/мл.
Другие компоненты: человеческий альбумин, хлорид натрия, вода для инъекций.
После смешивания | 1 мл | 2 мл | 4 мл | 10 мл |
Компонент 1: Раствор белка-клея Человеческий фибриноген (как коагулируемый белок) Синтетическая апротинина | 45,5 мг 1500 УИК | 91 мг 3000 УИК | 182 мг 6000 УИК | 455 мг 15000 УИК |
Компонент 2: Раствор тромбина Человеческая тромбина Хлорид кальция дигидрат | 250 ЕД 20 мкмоль | 500 ЕД 40 мкмоль | 1000 ЕД 80 мкмоль | 2500 ЕД 200 мкмоль |
ТИССЕЛ содержит 0,6–5 УИК/мл человеческого фактора XIII, который изолируется из плазмы вместе с человеческим фибриногеном.
Внешний вид продукта и содержимое упаковки
Растворы для тканевого клея.
Раствор белка-клея и раствор тромбина поставляются в шприце для одноразового использования.
Замороженные растворы бесцветны или светло-желтые и опалесцирующие.
После размораживания: жидкости бесцветны или светло-желтые.
ТИССЕЛ поставляется в следующей упаковке:
Содержимое упаковки с шприцем PRIMA:
- 1 мл, 2 мл или 5 мл раствора белка-клея и 1 мл, 2 мл или 5 мл раствора тромбина, содержащиеся в предварительно заполненном двукамерном шприце (полипропилен) с резьбовым крышком, упакованные в две сумки и с устройством с двумя соединительными частями и 4 наконечниками для применения.
Содержимое упаковки с шприцем AST:
- 1 мл, 2 мл или 5 мл раствора белка-клея и 1 мл, 2 мл или 5 мл раствора тромбина, содержащиеся в предварительно заполненном двукамерном шприце (полипропилен) с резьбовым крышком, упакованные в две сумки и с устройством с двумя соединительными частями, 4 наконечниками для применения и двойным поршневым эмболо.
Размеры упаковки:
ТИССЕЛ доступен в следующих размерах упаковки: 1 х 2 мл (1 мл + 1 мл), 1 х 4 мл (2 мл + 2 мл) и 1 х 10 мл (5 мл + 5 мл).
Растворы заморожены.
Возможно, не все размеры упаковки будут продаваться.
Владелец разрешения на маркетинг и ответственный за производство:
Владелец разрешения на маркетинг:
BAXTER, S.L.
Pouet de Camilo, 2
46394 Ribarroja del Turia (Valencia)
Ответственный за производство:
Takeda Manufacturing Austria AG,
Industriestrasse 67,
1221 Vienna, Austria
Это лекарство разрешено в государствах-членах Европейского экономического пространства под следующими названиями:
Австрия:TISSEEL - Lösungen für einen Gewebekleber
Болгария:T???? - ???????? ?? ??????? ??????
Чехия:TISSEEL - roztoky pro lepidlo
Франция:TISSEEL –solutions pour colle
Германия:TISSEEL 2ml
TISSEEL 4ml
TISSEEL 10ml
Греция:TISSEEL - Διαλ?ματα για στεγανοποιητικ?
Мальта:TISSEEL – Solutions for sealant
Норвегия:TISSEEL
Польша:TISSEEL - klej tkankowy
Словакия:TISSEEL – fibrinové lepidlo
Испания:TISSEEL – soluciones para adhesivo tisular
Дата последнего пересмотра этого листка-вкладыша: август 2020
Подробная информация о этом лекарстве доступна на сайте Агентства по лекарствам и медицинским изделиям (AEMPS) (http://www.aemps.gob.es/)
Эта информация предназначена только для медицинских специалистов (конечная упаковка: шприц PRIMA):
Общее
- Перед применением ТИССЕЛ необходимо покрыть все части тела вне области, подлежащей лечению, чтобы предотвратить прилипание тканей в нежелательных зонах.
- Чтобы избежать прилипания ТИССЕЛ к перчаткам и хирургическим инструментам, их необходимо смочить раствором хлорида натрия до контакта.
- Руководство по герметизации поверхностей: один контейнер ТИССЕЛ 2 мл (1 мл раствора белка-герметика и 1 мл раствора тромбина) достаточно для поверхности не менее 10 см2.
- Необходимая доза будет зависеть от размера поверхности, подлежащей герметизации.
- НЕ наносить отдельно два компонента ТИССЕЛ. Оба компонента должны быть нанесены вместе.
- НЕ подвергать ТИССЕЛ воздействию температур выше 37 °C и НЕ нагревать в микроволновой печи.
- НЕ размораживать продукт, держа его в руках.
- НЕ использовать ТИССЕЛ, пока он не будет полностью разморожен и нагрет до 33-37 °C.
- Защитные колпачки шприца должны быть удалены только после завершения размораживания и нагрева. Чтобы облегчить удаление колпачка шприца, его необходимо слегка покачать вперед и назад, а затем удалить защитный колпачок шприца.
- Удалить весь воздух из шприца и подключить соединительную насадку и насадку для нанесения.
Инструкции по обращению и подготовке
И раствор белка-герметика, и раствор тромбина находятся в шприце, готовом к использованию. Продукт упакован в две стерильные пакеты в асептических условиях. Внутренний пакет и его содержимое стерильны, пока внешний пакет не будет поврежден. С помощью стерильной техники необходимо перенести внутренний стерильный пакет и его содержимое в стерильное поле.
Шприц, готовый к использованию, можно разморозить и нагреть одним из следующих методов:
- Быстрая разморозка/нагрев (стерильная водяная баня),рекомендуемый метод
- Разморозка/нагрев в нестерильной водяной бане
- Разморозка/нагрев в инкубаторе
- Шприц, готовый к использованию, также можно разморозить и хранить при комнатной температуре (не выше 25 °C) до 72 часов. Перед использованием его необходимо нагреть.
- Быстрая разморозка/нагрев (стерильная водяная баня), рекомендуемый метод
Рекомендуется размораживать и нагревать два компонента тканевого клея, используя стерильную водяную баню при температуре 33-37 °C.
- Водяная баня не должна превышать 37 °C. Чтобы контролировать указанный диапазон температуры, необходимо отслеживать температуру воды с помощью термометра и менять воду при необходимости.
- Если используется стерильная водяная баня для размораживания и нагрева, необходимо удалить шприц из пакетов перед помещением его в стерильную водяную баню.
Инструкции:
Поместить внутренний пакет в стерильное поле, вынуть шприц, готовый к использованию, из внутреннего пакета и непосредственно поместить его в стерильную водяную баню. Обеспечить полное погружение содержимого шприца в воду.
Таблица 1: Шприц PRIMA: Минимальные времена размораживания и нагрева с использованием стерильной водяной бани
Размер упаковки | Минимальные времена размораживания/нагрева Стерильная водяная баня 33 °C - 37 °CПродукт, удаленный из пакетов |
2 мл | 5 минут |
4 мл | 5 минут |
10 мл | 10 минут |
- Разморозка/нагрев в нестерильной водяной бане
Инструкции:
Оставить шприц, готовый к использованию, в обеих пакетах и поместить его в нестерильную водяную баню вне стерильного поля на достаточный период времени (см. Таблицу 2). Обеспечить, чтобы пакеты оставались погруженными в воду на протяжении всего периода размораживания. После размораживания удалить пакеты из водяной бани, высушить внешний пакет и поместить внутренний пакет с шприцем в стерильное поле.
Таблица 2: Шприц PRIMA: Минимальные времена размораживания и нагрева с использованием нестерильной водяной бани
Размер упаковки | Минимальные времена размораживания/нагрева Нестерильная водяная баня 33 °C - 37 °CПродукт в пакетах |
2 мл | 15 минут |
4 мл | 20 минут |
10 мл | 35 минут |
- Разморозка/нагрев в инкубаторе
Инструкции:
Оставить шприц, готовый к использованию, в обеих пакетах и поместить его в инкубатор вне стерильного поля на достаточный период времени (см. Таблицу 3). После размораживания/нагрева удалить пакеты из инкубатора, удалить внешний пакет и поместить внутренний пакет с шприцем в стерильное поле.
Таблица 3: Шприц PRIMA: Минимальные времена размораживания и нагрева в инкубаторе
Размер упаковки | Минимальные времена размораживания/нагрева 33 °C - 37 °C в инкубаторе Продукт в пакетах |
2 мл | 40 минут |
4 мл | 50 минут |
10 мл | 90 минут |
- Разморозка при комнатной температуре (не выше 25 °C) ДО нагрева
Инструкции:
Оставить шприц, готовый к использованию, в обеих пакетах и разморозить его при комнатной температуре вне стерильного поля на достаточный период времени (см. Таблицу 4). После размораживания нагреть его в внешнем пакете в инкубаторе для нагрева продукта перед использованием.
Таблица 4: Шприц PRIMA: Минимальные времена размораживания при комнатной температуре вне стерильного поля и дополнительные времена нагрева в инкубаторе 33 °C - 37 °C
Размер упаковки | Минимальные времена размораживания продукта при комнатной температуре (не выше 25 °C) Продукт в пакетах | Времена предварительного нагрева перед использованием при 33 °C - 37 °C в инкубаторе после размораживания при комнатной температуре Продукт в пакетах |
2 мл | 80 минут + 11 минут | |
4 мл | 90 минут + 13 минут | |
10 мл | 160 минут + 25 минут |
После размораживания при комнатной температуре продукт должен быть использован в течение 72 часов после его удаления из холодильника.
Стабильность после размораживания
После размораживанияи нагрева(при температурах между 33 °C и 37 °C, методы 1, 2 и 3) была продемонстрирована химическая и физическая стабильность продукта в течение 12 часов при 33-37 °C.
В случае продукта, размороженногопри комнатной температуре, в неповрежденной упаковке (метод 4), была продемонстрирована химическая и физическая стабильность продукта в течение 72 часов при температурах не выше 25 °C. Нагреть до 33-37 °C непосредственно перед использованием.
С микробиологической точки зрения, если метод открытия/размораживания исключает риск микробной контаминации, продукт должен быть использован сразу после нагрева до 33-37 °C.
Если он не используется сразу, сроки и условия хранения во время использования являются ответственностью пользователя.
НЕ повторно замораживать или охлаждать после начала размораживания.
Обращение после размораживания/до применения
Чтобы достичь оптимального смешивания двух растворов и оптимального затвердевания тканевого клея фибрина, поддерживатьоба компонента тканевого клея при 33-37 °C до применения.
Растворы белка-герметика и тромбина должны быть прозрачными или слегка опалесцирующими. Не использовать растворы, которые являются мутными или имеют осадки. Размораженный продукт должен быть визуально осмотрен перед использованием, чтобы исключить наличие частиц, изменение цвета или любые изменения внешнего вида. Если какие-либо из этих обстоятельств наблюдаются, растворы должны быть выброшены.
Размораженный раствор белка-герметика должен быть слегка вязкой жидкостью. Если раствор имеет консистенцию затвердевшего геля, можно предположить, что он денатурировался (возможно, из-за нарушения холодовой цепи или избыточного нагрева во время нагрева). В этом случае ТИССЕЛ НЕ должен быть использован ни в коем случае.
- Удалить шприц из пакетов непосредственно перед использованием.
- Использовать ТИССЕЛ только после полного размораживания и нагрева (жидкая консистенция).
- Удалить защитный колпачок шприца непосредственно перед применением.
Чтобы облегчить удаление колпачка шприца, его необходимо слегка покачать вперед и назад, а затем удалить защитный колпачок шприца.
Применениес шприцем PRIMA:
Для применения шприц с двойной камерой, готовый к использованию, с растворами белка-герметика и тромбина должен быть подключен к соединительной насадке и насадке для нанесения, которые входят в состав набора устройств для применения. Часть, соединяющая на конце поршни шприца с двойной камерой, готового к использованию, гарантирует, что равные объемы двух компонентов тканевого клея будут выходить через соединительную насадку в насадку для нанесения, где они будут смешиваться перед применением.
Инструкции по эксплуатации шприца PRIMA:
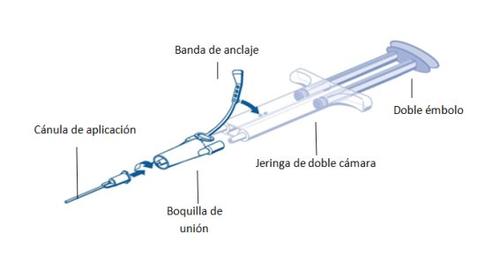
- Удалить весь воздух из шприца перед подключением любого устройства для применения.
- Соединить соединительную насадку и закрепить ее на боку шприца в отверстии зоны крепления.
- Подключить насадки шприца с двойной камерой, готового к использованию, к соединительной насадке, убедившись, что они надежно закреплены.
- Закрепить соединительную насадку, зафиксировав зону крепления на шприце с двойной камерой, готовом к использованию.
- Если зона крепления разрывается, использовать запасную соединительную насадку, входящую в состав набора.
- Если запасная соединительная насадка недоступна, все равно можно использовать систему, если соединение надежное и герметичное.
- НЕ удалять воздух, оставшийся в соединительной насадке.
- Подключить насадку для нанесения к соединительной насадке.
- НЕ удалять воздух, оставшийся в соединительной насадке и насадке для нанесения, до начала применения, поскольку это может засорить отверстие насадки.
Применение
Перед применением ТИССЕЛ необходимо высушить поверхность раны, используя стандартную технику (например, периодическое применение компрессов, тампонов, использование аспирационных устройств). Не использовать сжатый воздух или газ для сушки области.
- Нанести смесь раствора белка-герметика и раствора тромбина на поверхность или поверхности частей, подлежащих герметизации, медленно нажимая на заднюю часть общего поршня.
- В хирургических процедурах, требующих минимальных объемов тканевого клея фибрина, рекомендуется удалить и выбросить первые капли продукта.
- После применения ТИССЕЛ подождать не менее 2 минут, чтобы обеспечить достаточную полимеризацию.
Примечание:Если применение компонентов тканевого клея фибрина прерывается, могут образовываться сгустки в насадке. В этом случае необходимо немедленно заменить насадку для нанесения на новую перед возобновлением применения. Если отверстия соединительной насадки засорены, использовать дополнительную соединительную насадку, входящую в состав упаковки.
После смешивания компонентов тканевого клея фибриновый клей начинает затвердевать в течение нескольких секунд из-за высокой концентрации тромбина (500 ЕД/мл).
Тканевой клей фибрина также можно наносить с помощью других аксессуаров, поставляемых компанией BAXTER, которые особенно подходят для, например, эндоскопического использования, минимально инвазивной хирургии или нанесения на большие или труднодоступные области. При использовании этих устройств для нанесения необходимо внимательно следовать их инструкциям по использованию.
НЕ использовать препараты, содержащие окисленную целлюлозу, с ТИССЕЛ, поскольку низкий pH нарушает активность тромбина.
В некоторых применениях используется биосовместимый материал, такой как коллагеновые листы, в качестве поддерживающего материала или для укрепления.
Нанесение методом распыления
При нанесении ТИССЕЛ с помощью распылительного оборудования необходимо обеспечить, чтобы давление и расстояние до ткани находились в рекомендуемых производителем диапазонах, как указано ниже:
Давление, расстояние и рекомендуемое оборудование для нанесения ТИССЕЛ методом распыления | |||||
Хирургическая процедура | Распылительное оборудование, которое должно быть использовано | Насадки для нанесения, которые должны быть использованы | Регулятор давления, который должен быть использован | Рекомендуемое расстояние до целевой ткани | Рекомендуемое давление распыления |
Открытая рана | Распылительное оборудование ТИССЕЛ/Артисс | н.а. | ИзиСпрей | 10-15 см | 1,5-2,0 бар (21,5-28,5 psi). |
Распылительное оборудование ТИССЕЛ/Артисс, упаковка 10 | н.а. | ИзиСпрей | |||
Лапароскопические или минимально инвазивные процедуры | н.а. | Насадка ДуплоСпрей МИ 20 см | Регулятор ДуплоСпрей МИ 1,5 бар | 2-5 см | 1,2-1,5 бар (18-22 psi) |
Насадка ДуплоСпрей МИ 30 см | |||||
Насадка ДуплоСпрей МИ 40 см | |||||
Эндоскопический набор для распыления 360 со снэп-локом | |||||
Эндоскопический набор для распыления 360 с креплением | |||||
Заменяемая насадка |
Всегда, когда ТИССЕЛ наносится методом распыления, и поскольку существует возможность газовой эмболии (воздух или газ), необходимо контролировать изменения артериального давления, пульса, насыщения кислородом и уровня CO2в конце выдоха(см. раздел 2).
Для нанесения ТИССЕЛ в закрытые грудную и брюшную полости рекомендуется использовать систему нанесения и регулятор ДуплоСпрей МИ. Прочитайте руководство по устройству ДуплоСпрей МИ.
Утилизация
Утилизация неиспользованного лекарственного средства и материалов отходов должна осуществляться в соответствии с местными правилами.
- Страна регистрации
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги ТИССИЛ Раствор для тканевого клеяФорма выпуска: ТКАНЕВЫЙ КЛЕЙ, в зависимости от клинических потребностей пациентаАктивное вещество: combinationsПроизводитель: Baxter S.L.Требуется рецептФорма выпуска: ТКАНЕВЫЙ КЛЕЙ, 50-90 мг/мл 800-1200 МЕАктивное вещество: combinationsПроизводитель: Omrix BiopharmaceuticalsТребуется рецептФорма выпуска: ТКАНЕВЫЙ КЛЕЙ, -Активное вещество: combinationsПроизводитель: Corza Medical GmbhТребуется рецепт
Аналоги ТИССИЛ Раствор для тканевого клея в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог ТИССИЛ Раствор для тканевого клея в Україна
Врачи онлайн по ТИССИЛ Раствор для тканевого клея
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на ТИССИЛ Раствор для тканевого клея – по решению врача и с учетом местных правил.














