TIMOFTOL 5 mg/ml COLIRIO EN SOLUCION

Cómo usar TIMOFTOL 5 mg/ml COLIRIO EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
TIMOFTOL 5 mg/ml colirio en solución
Timolol
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es TIMOFTOL y para qué se utiliza
- Qué necesita saber antes de empezar a usar TIMOFTOL
- Cómo usar TIMOFTOL
- Posibles efectos adversos
- Conservación de TIMOFTOL
- Contenido del envase e información adicional
1. Qué es TIMOFTOL y para qué se utiliza
Timoftol es un agente betabloqueante oftálmico que pertenece al grupo de medicamentos denominado antiglaucomatosos tópicos.
Este medicamento está indicado para reducir la presión elevada en el ojo en:
- hipertensión ocular
- glaucoma crónico de ángulo abierto (incluyendo pacientes afáquicos)
- algunos pacientes con glaucoma secundario.
2. Qué necesita saber antes de empezar a usar TIMOFTOL
No use TIMOFTOL
- Si es alérgico (hipersensible) al timolol, a los betabloqueantes o a cualquiera de los demás componentes de Timoftol (incluidos en la sección 6).
- Si tiene ahora o ha tenido en el pasado ciertos problemas respiratorios importantes tales como asma.
- Si tiene ahora o ha tenido en el pasado enfermedad pulmonar obstructiva crónica (enfermedad grave de los pulmones que puede ocasionar respiración sibilante, dificultad respiratoria y/o tos persistente).
- Si tiene ciertas enfermedades cardiacas (como latidos del corazón lentos o irregulares), bradicardia sinusal (frecuencia cardiaca inferior a 60 latidos por minuto), bloqueo aurículo ventricular de segundo o tercer grado, insuficiencia cardiaca manifiesta o shock cardiogénico.
- Si tiene distrofia corneal (alteración degenerativa de la córnea).
- Si tiene rinitis alérgica grave e hiperreactividad bronquial.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Timoftol.
Tenga especial cuidado con Timoftol.
Antes de utilizar este medicamento, informe a su médico si tiene o ha tenido en el pasado:
- problemas del corazón como enfermedad coronaria (los síntomas incluyen dolor torácico u opresión, falta de aire o ahogo), insuficiencia cardiaca o tensión arterial baja
- alteraciones del ritmo del corazón (como latidos del corazón lentos o irregulares)
- problemas de la circulación (como la enfermedad de Raynaud o el síndrome de Raynaud)
- problemas respiratorios o de pulmón (como asma o enfermedad pulmonar obstructiva crónica)
- diabetes u otros problemas de azúcar en la sangre, ya que el timolol puede enmascarar los signos y síntomas de bajo nivel de azúcar en sangre
- sobreactividad o enfermedad de la tiroides, ya que el timolol puede enmascarar sus signos y síntomas
Dígale a su médico, antes de una intervención quirúrgica, que está usando Timoftol, ya que puede cambiar los efectos de algunos medicamentos usados durante la anestesia.
También informe a su médico sobre cualquier alergia o medicación.
Timolol puede absorberse a través del ojo llegando a sangre, pueden producirse los mismos efectos adversos que con la administración de otros fármacos betabloqueantes orales.
- Si está tomando medicamentos betabloqueantes por vía oral o medicamentos antidepresivos inhibidores de la monoaminooxidasa debe informar a su médico, ya que podrían aumentar los efectos de Timoftol.
- No se recomienda usar dos medicamentos betabloqueantes tópicos al mismo tiempo.
- Si padece enfermedad del seno, angina de Prinzmetal, feocromocitoma no tratado, acidosis metabólica, trastornos circulatorios periféricos graves (enfermedad de Raynaud) o tensión arterial baja.
- Si utiliza lentes de contacto, no se recomienda el uso de Timoftol, ya que aumenta el riesgo de presentar intolerancia a las mismas.
Como con cualquier tratamiento para el glaucoma, es recomendable que su médico controle regularmente la presión del ojo y el estado de la córnea.
Uso en deportistas
Este medicamento contiene timolol que puede producir un resultado positivo en las pruebas de control de dopaje.
Niños
Como norma general, se deben utilizar con precaución los colirios conteniendo timolol en solución en los pacientes jóvenes. En el caso de recién nacidos, lactantes y niños de corta edad, el timolol se debe utilizar con extrema precaución. Se debe interrumpir inmediatamente el uso de este medicamento en el caso de aparecer tos, respiración sibilante, respiración anormal o paradas anormales de la respiración (apnea). Informe inmediatamente a su médico. Puede ser de utilidad un controlador portátil de apnea.
Se ha estudiado el timolol en lactantes y niños de edades comprendidas entre 12 días y 5 años en los que había un aumento de la presión del (de los) ojo(s) o en los que se había diagnosticado un glaucoma. Para más información, consulte a su médico.
Uso de TIMOFTOL con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento, incluso los adquiridos sin receta médica.
Ciertos medicamentos pueden interaccionar con Timoftol, incluyendo otros colirios para el tratamiento del glaucoma, y en estos casos puede ser necesario cambiar la dosis o interrumpir el tratamiento con cualquiera de ellos. Es importante que informe a su médico si toma alguno de los siguientes medicamentos:
- Betabloqueantes por vía oral o medicamentos que disminuyan la tensión arterial, ya que pueden aumentar los efectos de timolol sobre la presión intraocular.
- Clonidina, ya que puede producirse tensión arterial alta de rebote al interrumpir el tratamiento con clonidina.
- Medicamentos para el tratamiento del ritmo del corazón, como disopiramida, quinidina (utilizada también para tratar algunos tipos de malaria) y amiodarona, ya que timolol puede aumentar sus efectos.
- Medicamentos para la diabetes (insulina y antidiabéticos orales), ya que timolol puede enmascarar determinados signos de hipoglucemia (azúcar en sangre bajo) como taquicardia (ritmo rápido del corazón).
- Anestésicos.
- Medicamentos para la úlcera de estómago como cimetidina.
- Alcohol.
- Epinefrina, ya que junto con timolol puede producir dilatación de la pupila (midriasis).
- Medicamentos para la depresión, como fluoxetina o paroxetina.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No utilice Timoftol si usted está embarazada, a menos que su médico considere que sea necesario. Por la posible aparición de efectos adversos en el feto, su médico valorará el beneficio/riesgo de la administración de Timoftol.
No utilice Timoftol si está en período de lactancia. Timolol puede pasar a la leche materna. Debido a la posible aparición de efectos adversos, su médico decidirá si suspender el tratamiento con Timoftol o suspender la lactancia.
Conducción y uso de máquinas
Timoftol puede hacerle experimentar mareo, fatiga o visión borrosa, que pueden afectar a su capacidad para conducir y utilizar maquinaria.
TIMOFTOL contiene cloruro de benzalconio y fosfatos
Este medicamento contiene 0,11 mg de cloruro de benzalconio en cada ml.
El cloruro de benzalconio se puede absorber por las lentes de contacto blandas y puede alterar el color de las lentes de contacto. Retirar las lentes de contacto antes de usar este medicamento y. esperar 15 minutos antes de volver a colocarlas.
El cloruro de benzalconio puede causar irritación ocular, especialmente si padece de ojo seco u otras enfermedades de la córnea (capa transparente de la zona frontal del ojo). Consulte a su médico si siente una sensación extraña, escozor o dolor en el ojo después de usar este medicamento.
Este medicamento contiene 30,42 mg de hidrogenofosfato de disodio dodecahidrato y 6,10 mg de dihidrogenofosfato de sodio dihidrato en cada ml. Si sufre de daño grave en la capa trasparente de la parte frontal del ojo (córnea) el tratamiento con fosfatos, en casos muy raros, puede provocar parches nublados en la córnea debido al calcio.
3. Cómo usar TIMOFTOL
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Recuerde usar el medicamento.
Su médico establecerá la dosis y la duración del tratamiento con Timoftol. No suspenda el tratamiento antes de tiempo ya que cesaría su efecto beneficioso.
Timoftol es un colirio para administración por vía oftálmica.
La dosis normal es una gota de Timoftol 2,5 mg/ml en el ojo u ojos afectados dos veces al día. Si la respuesta no es satisfactoria, su médico podrá aumentar la dosis a una gota de Timoftol 5 mg/ml en el ojo u ojos afectados dos veces al día.
Su médico evaluará periódicamente la respuesta al tratamiento con Timoftol y decidirá si es necesario complementarlo con otros medicamentos disponibles para reducir la presión intraocular.
Si está utilizando a la vez otros colirios, deberá esperar al menos 10 minutos entre las aplicaciones para que los principios activos no se eliminen del ojo.
En el caso de que este colirio sustituya a otro tratamiento para el glaucoma anterior o se emplee junto con otros medicamentos, su médico le indicará la pauta que debe seguir.
Si estima que la acción de Timoftol es demasiado fuerte o débil, comuníqueselo a su médico o farmacéutico.
Uso en niños y adolescentes
Antes de la utilización de timolol, se debe realizar un examen médico completo. Su médico evaluará cuidadosamente los beneficios frente a los riesgos antes de plantear un tratamiento con timolol. Si los beneficios son superiores a los riesgos, se recomienda utilizar una vez al día la concentración más baja disponible de sustancia activa.
Si no se controla suficientemente la presión con esta concentración, puede ser necesaria la administración dos veces al día con 12 horas de intervalo entre ellas. Se debe controlar estrechamente a los pacientes, en especial los recién nacidos, durante una a dos horas posteriores después de la primera administración, vigilando con atención la aparición de efectos adversos hasta que se lleve a cabo la cirugía. En el caso de su uso en niños, para controlar la presión en el interior del ojo puede ser suficiente la concentración del 1 mg/ml en sustancia activa, en el caso de que estuviera disponible.
Duración del tratamiento
En la población pediátrica, se prescribirá como un tratamiento temporal.
Forma de administración
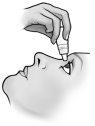
En cada administración, solamente se debe instilar una gota de Timoftol.
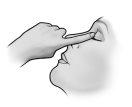
Tras la instilación, mantener los ojos cerrados tanto tiempo como sea posible (por ejemplo, 3 a 5 minutos) y mantener presionado con un dedo el ángulo del ojo más cercano a la nariz a fin de impedir la difusión de la gota de timolol al cuerpo.
Instrucciones de uso
No utilice el envase si la tira de seguridad de plástico alrededor del cuello del envase no está o está rota. Cuando abra el envase por primera vez, arranque la tira de seguridad de plástico.
[para envases distintos de OCUMETER PLUS:]
Cada vez que utilice Timoftol:
| |
| |
|
|
|
|
|
|
[solamente para envases OCUMETER PLUS:]
- Antes de utilizar el medicamento por primera vez, asegúrese de que la Tira de Seguridad en la parte delantera del frasco esté intacta. Cuando el frasco no se ha abierto aún, es normal la existencia de un espacio entre el frasco y el capuchón.
Flechas de Apertura ?
Tira de seguridad ?
- Lávese las manos. Cuando abra el frasco por primera vez, arranque la Tira de Seguridad para romper el precinto.
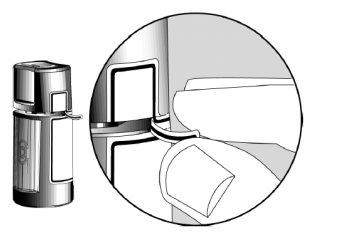
Espacio ?
Área para Presionar con el
Dedo ?
- Para abrir el frasco, desenroscar el capuchón girándolo según las indicaciones de las flechas de la parte de arriba del capuchón. No tire directamente del capuchón del frasco hacia arriba. Tirar del capuchón hacia arriba impedirá que el dispensador funcione correctamente.

Área para Presionar con el
Dedo ?
- Incline la cabeza hacia atrás y separe el párpado inferior ligeramente, formando una pequeña separación entre el párpado y el ojo.
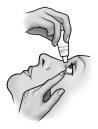
- Invierta el frasco y presione ligeramente con el dedo pulgar o con el índice sobre el “Área para Presionar Con el Dedo” (como muestra la siguiente figura) hasta dispensar una sola gota en el ojo de acuerdo con las instrucciones de su médico.
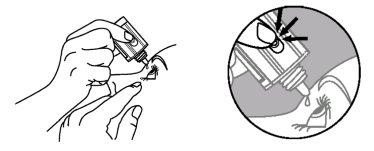
Área para Presionar
con el Dedo
NO TOQUE EL OJO NI EL PÁRPADO CON LA PUNTA DEL TAPÓN CUENTAGOTAS.
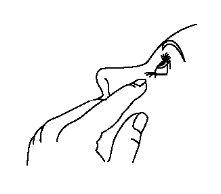
- Después de usar Timoftol presione con el dedo en la esquina del ojo al lado de la nariz (como muestra la siguiente figura) durante 2 minutos. Esto ayuda a mantener Timoftol en el ojo e impide la difusión de timolol al resto del cuerpo.
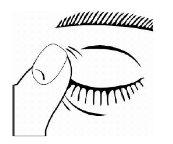
- Si después de abrir por primera vez, la dispensación de la gota es dificultosa, coloque de nuevo el capuchón y apriete (NO APRIETE DEMASIADO) y luego quite el capuchón girándolo en la dirección contraria, como está indicado en las flechas de la parte de arriba del capuchón.
- Repita los pasos 4 y 5 en el otro ojo si así se lo ha indicado su médico.
- Cierre el capuchón girándolo hasta que toque el borde del frasco. Para un cierre adecuado, la flecha del lado izquierdo del capuchón debe estar alineada con la flecha del lado izquierdo de la etiqueta del frasco. No lo apriete demasiado o puede estropear el frasco y el capuchón.
- La punta del dispensador está diseñada para proporcionar una única gota; por tanto, NO ensanche el agujero de la punta del dispensador.
- Después de que haya usado todas las dosis, quedará algo de Timoftol en el frasco. No debe preocuparse ya que se ha añadido una cantidad extra de Timoftol y usted obtendrá la cantidad total de Timoftol que su médico le ha recetado. No intente extraer el exceso de medicamento del frasco.
Los medicamentos oftálmicos, si se utilizan inadecuadamente, pueden contaminarse con bacterias comunes conocidas por causar infecciones en los ojos. El uso de soluciones oftálmicas contaminadas puede dar lugar a trastornos oculares graves y la subsiguiente pérdida de la visión. Si piensa que su medicación puede estar contaminada, o si desarrolla una infección en el ojo, contacte con su médico inmediatamente sobre el uso continuado de ese frasco.
Si usa más TIMOFTOL del que debe
Si ha utilizado más Timoftol de lo que debe, consulte inmediatamente a su médico o a su farmacéutico.
Los síntomas más comunes en caso de sobredosis con timolol son: mareo, cefalea, respiración entrecortada, disminución del número de latidos cardiacos, descenso de la tensión arterial, insuficiencia cardiaca y/o paro cardiaco.
En caso de sobredosis o ingestión accidental consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91.562.04.20, indicando el medicamento y la cantidad utilizada.
Si olvidó usar TIMOFTOL
No use una dosis doble para compensar las dosis olvidadas.
Use Timoftol, según la pauta que le haya indicado su médico. Si olvida una dosis, adminístrela lo antes posible. Sin embargo, si ya es casi la hora de la siguiente dosis, ignore la dosis olvidada y vuelva a su pauta de administración habitual.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Timoftol puede producir efectos adversos, aunque no todas las personas los sufran.
Normalmente podrá seguir utilizando las gotas, a menos que los efectos sean graves. Si está preocupado, consulte a su médico o farmacéutico. No interrumpa el uso de Timoftol sin comentarlo antes con su médico.
Con la administración de timolol por vía oftálmica se han observado los siguientes efectos adversos:
Frecuentes (pueden afectar hasta 1 de cada 10 pacientes):
- dolor de cabeza
- signos y síntomas de irritación ocular (p.ej., sensación de quemazón, pinchazos, picor, conjuntivitis, lagrimeo, enrojecimiento), inflamación de los párpados, inflamación de la córnea, disminución de la sensibilidad corneal y ojos secos
Poco frecuentes (pueden afectar hasta 1 de cada 100 pacientes):
- depresión
- mareos, desmayo
- trastornos visuales como cambios refractivos
- ritmo cardiaco lento (bradicardia)
- dificultad en la respiración (disnea)
- náuseas, digestión pesada (dispepsia)
- cansancio /fatiga
Raros (pueden afectar hasta 1 de cada 1.000 pacientes):
- signos y síntomas de reacciones alérgicas incluyendo angioedema (hinchazón debajo de la piel en zonas como la cara y las extremidades, y que puede obstruir las vías respiratorias y puede causar dificultad al tragar o respirar), urticaria o sarpullido con picor, erupción localizada y generalizada, anafilaxia (reacción alérgica grave que puede poner en peligro la vida)
- insomnio (dificultad para dormir), pesadillas, pérdida de memoria
- sensación de hormigueo o agujetas, aumento en los signos y síntomas de miastenia gravis (trastorno muscular), disminución del apetito sexual (libido disminuida), accidente cerebrovascular (ictus), isquemia cerebral (llegada reducida de sangre al cerebro)
- párpado superior caído (quedando el ojo medio cerrado), visión doble (diplopía), desprendimiento coroideo (desprendimiento de la capa vascular debajo de la retina después de la cirugía de filtración que puede causar alteraciones en la visión)
- ruidos en los oídos (acúfenos)
- dolor torácico, palpitaciones, hinchazón, ritmo cardiaco irregular, insuficiencia cardiaca congestiva (enfermedad caracterizada por dificultad para respirar e hinchazón de pies y piernas debido a la acumulación de líquidos), bloqueo cardiaco, parada cardiaca
- tensión arterial baja, fenómeno de Raynaud (trastorno de los vasos sanguíneos que afecta generalmente a los dedos de manos y pies), dolor o molestias en una extremidad al comenzar a caminar, manos y pies fríos
- problemas al respirar y constricción de las vías respiratorias (predominante en pacientes con enfermedad broncoespástica pre-existente), tos
- diarrea, boca seca
- pérdida de pelo, erupción cutánea psoriasiforme o exacerbación de psoriasis
- enfermedad inflamatoria con fiebre, debilidad, dolor en las articulaciones y lesiones en la piel (lupus eritematoso sistémico)
- enfermedad de Peyronie (que puede causar una marcada curvatura del pene)
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles):
- alucinaciones
Como los otros medicamentos que se aplican en los ojos, el timolol pasa a la sangre. Esto puede ocasionar efectos adversos similares a los observados con los agentes betabloqueantes administrados por vía oral o inyectables. La aparición de efectos adversos por vía tópica oftálmica es menos frecuente que en el caso de administración por vía oral o inyectable. Los efectos adversos listados incluyen aquellos que se han observado en la clase de los betabloqueantes utilizados para tratar las enfermedades oculares:
- Reacciones alérgicas generalizadas incluyendo hinchazón debajo de la piel en zonas como la cara y las extremidades, y que puede obstruir las vías respiratorias y puede causar dificultad al tragar o respirar, urticaria o sarpullido con picor, erupción localizada y generalizada, picor, reacción alérgica grave que puede poner en peligro la vida.
- Nivel bajo de glucosa en sangre.
- Dificultad para dormir (insomnio), depresión, pesadillas, pérdida de memoria.
- Desmayo, accidente cerebrovascular (ictus), reducción del aporte sanguíneo al cerebro, empeoramiento de los signos y síntomas de miastenia gravis (trastorno muscular), mareo, sensaciones extrañas como agujetas y dolor de cabeza.
- Signos y síntomas de irritación ocular (p. ej., quemazón, pinchazos, picor, lagrimeo, enrojecimiento), inflamación del párpado, inflamación de la córnea, visión borrosa y desprendimiento de la capa vascular debajo de la retina después de la cirugía de filtración que puede causar alteraciones en la visión, disminución de la sensibilidad corneal, ojos secos, erosión corneal (daño en la capa frontal del globo ocular), párpado superior caído (quedando el ojo medio cerrado), visión doble.
- Ritmo cardiaco lento, palpitaciones, dolor torácico, edema (aumento de líquido), cambios en la velocidad o ritmo del latido cardiaco, insuficiencia cardiaca congestiva (enfermedad caracterizada por dificultad para respirar e hinchazón de pies y piernas debido a la acumulación de líquidos), algún trastorno en el ritmo cardiaco, bloqueo cardiaco, parada cardiaca
- Tensión arterial baja, fenómeno de Raynaud, manos y pies fríos.
- Constricción de las vías respiratorias (predominante en pacientes con enfermedad pre-existente), dificultad para respirar, tos.
- Alteración del gusto, náuseas, indigestión, diarrea, boca seca, dolor abdominal, vómitos.
- Pérdida de pelo, erupción cutánea de color blanco-plateado (erupción psoriasiforme) o empeoramiento de la psoriasis, erupción cutánea.
- Dolor muscular no causado por ejercicio.
- Disfunción sexual, disminución de la libido.
- Debilidad muscular/cansancio.
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es.
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de TIMOFTOL
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 25 ºC.
Conservar en el embalaje original para protegerlo de la luz.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de la abreviatura CAD.
La fecha de caducidad es el último día del mes que se indica.
Desechar a las cuatro semanas tras la apertura del envase.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de TIMOFTOL
- El principio activo es timolol. Cada ml de Timoftol contiene 6,8 mg de maleato de timolol, equivalentes a 5 mg de timolol
- Los demás componentes son dihidrogenofosfato de sodio dihidrato, hidrogenofosfato de disodio dodecahidrato, hidróxido de sodio, cloruro de benzalconio y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Timoftol se presenta en forma de colirio en solución transparente, incolora o amarillo claro.
Se presenta en dos envases alternativos:
- Envase con tapón cuentagotas que contiene 5 ml de solución.
- Dispensador oftálmico Ocumeter Plus que contiene 3 ml de solución.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Santen Oy
Niittyhaankatu 20
33720 Tampere
Finlandia
Responsable de la fabricación
Laboratoires Merck Sharp & Dohme
Chibret (“MIRABEL PLANT”)
Route de Marsat, RIOM
63963 Clermont – Ferrand, Cedex 9, Francia
Ó
Santen Oy
Kelloportinkatu 1
33100 Tampere
Finlandia
Representante local
Santen Pharmaceutical Spain S.L.
Acanto, 22, 7º
28045 – Madrid
España
Fecha de la última revisión de este prospecto: Abril 2020
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TIMOFTOL 5 mg/ml COLIRIO EN SOLUCIONForma farmacéutica: COLIRIO, 5 mg/mlPrincipio activo: timololFabricante: Immedica Pharma AbRequiere recetaForma farmacéutica: GEL OFTALMICO, 1 mg/gPrincipio activo: timololFabricante: Sifi S.P.A.Requiere recetaForma farmacéutica: COLIRIO, 0,25%Principio activo: timololFabricante: Laboratorios Thea S.A.Requiere receta
Médicos online para TIMOFTOL 5 mg/ml COLIRIO EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TIMOFTOL 5 mg/ml COLIRIO EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes