TEGSEDI 284 mg Injectable Solution in Pre-filled Syringe

How to use TEGSEDI 284 mg Injectable Solution in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Tegsedi 284 mg solution for injection in pre-filled syringe
Inotersen
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Tegsedi and what is it used for
- What you need to know before you use Tegsedi
- How to use Tegsedi
- Possible side effects
- Storage of Tegsedi
- Contents of the pack and other information
1. What is Tegsedi and what is it used for
Tegsedi contains the active substance inotersen. Inotersen is used to treat adults with hereditary transthyretin-mediated amyloidosis. Hereditary transthyretin-mediated amyloidosis is a genetic disease that causes the accumulation of small fibers of a protein called transthyretin in the organs of your body, preventing them from working properly. Tegsedi is used when the disease causes symptoms of polyneuropathy (nerve damage).
The active substance in Tegsedi, inotersen, is a type of medicine called an antisense oligonucleotide inhibitor. It works by reducing the production of transthyretin by the liver, and thus reduces the risk of transthyretin fibers being deposited in the organs and causing symptoms.
2. What you need to know before you use Tegsedi
Do not use Tegsedi:
- if you are allergic to inotersen or any of the other ingredients of this medicine (listed in section 6).
- if the tests indicate that you have a very low platelet count (the cells in the blood that help the blood to clot).
- if the kidney function tests or protein in urine tests show signs of severe kidney problems.
- if you have severe liver function impairment (liver failure).
Warnings and precautions
Before starting treatment with Tegsedi, your doctor will check your blood cells, liver function, kidney function, vitamin A, and protein levels in the urine. They may also do a pregnancy test to make sure you are not pregnant. Unless your doctor recommends it, you will only be treated with Tegsedi if all these test results are acceptable, and your pregnancy test result is negative. Your doctor will repeat these tests regularly during treatment. It is important that you have these blood and urine tests regularly while you are taking Tegsedi.
Thrombocytopenia
Tegsedi may reduce the blood cells responsible for clotting (platelets), which can lead to a condition called thrombocytopenia at any time during treatment with Tegsedi (see section 4). When you do not have enough platelets, as in thrombocytopenia, your blood may not clot quickly enough to stop bleeding. This can cause bruising, as well as more serious problems such as excessive bleeding or internal bleeding. Your doctor will do blood tests to check your platelet levels before treatment and regularly during treatment with Tegsedi. It is important that you have these blood tests regularly while you are taking Tegsedi due to the risk of serious bleeding caused by low platelet counts. If you stop using Tegsedi, your blood values should be checked 8 weeks after stopping the medicine.
If you notice unexplained bruising or a rash of small red spots that appear on the skin (called petechiae), bleeding from cuts in the skin that do not stop, nosebleeds or bleeding gums, blood in the urine or stools, or bleeding in the white part of the eyes, you should contact your doctor immediately. You should seek immediate help if you have a stiff neck or an unusual and severe headache, as these symptoms can be caused by a brain bleed.
Glomerulonephritis/kidney problems
Glomerulonephritis is a condition of the kidneys, in which they do not work properly due to inflammation and kidney damage. Some patients treated with inotersen have developed this condition. The symptoms of glomerulonephritis are foam in the urine, urine that is pink or brown, blood in the urine, and urinating less than usual.
Some patients treated with inotersen have also developed a decline in kidney function without having glomerulonephritis.
Your doctor will check your kidney function before treatment and regularly during treatment with Tegsedi. It is essential that you have these blood tests regularly while you are taking Tegsedi due to the risk of serious bleeding caused by low platelet counts. If you stop using Tegsedi, your kidney function should be checked 8 weeks after stopping the medicine. If you develop glomerulonephritis, your doctor will treat you for this condition.
Vitamin A deficiency
Tegsedi may decrease the levels of vitamin A (also called retinol) in your body. Your doctor will measure these levels, and if they are already low, this should be corrected and all symptoms should be resolved before starting treatment with Tegsedi. The symptoms of low vitamin A include:
- Dry eyes, poor vision, decreased night vision, blurred or cloudy vision
If you have any problems with your vision or any other problems with your eyes while taking Tegsedi, you should inform your doctor. Your doctor may refer you to an eye specialist for a check-up if necessary.
Your doctor will ask you to take a daily vitamin A supplement during treatment with Tegsedi.
Both high and low levels of vitamin A can harm the development of the unborn child. Therefore, women of childbearing age must rule out pregnancy before starting treatment with Tegsedi and must use effective contraceptive methods (see section "Pregnancy and breastfeeding" below).
If you plan to become pregnant, you will stop taking inotersen, including vitamin A supplements, and check that your vitamin A levels have returned to normal before trying to conceive.
If you have an unplanned pregnancy, you will stop taking inotersen. However, due to the prolonged activity of Tegsedi, your reduced vitamin A levels may persist. It is not known whether continuing vitamin A supplementation at a dose of 3000 IU per day will be harmful to your unborn child during the first trimester of your pregnancy, but this dose should not be exceeded. You will resume vitamin A supplementation during the second and third trimesters of your pregnancy if your vitamin A levels have not returned to normal, due to the higher risk of vitamin A deficiency in the third trimester.
Liver damage and liver monitoring
Tegsedi may cause severe liver problems. Before starting to take inotersen, you will need to have a blood test to check that your liver is working properly. You will also need to have these blood tests regularly while taking this medicine. It is essential that you have these blood tests regularly while taking Tegsedi.
Liver transplant rejection
Consult your doctor before using Tegsedi if you have had a liver transplant. There have been reports of liver transplant rejection in patients treated with Tegsedi. Your doctor will monitor this aspect regularly during treatment with Tegsedi.
Children and adolescents
Tegsedi should not be used in children and adolescents under 18 years of age.
Other medicines and Tegsedi
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. It is essential that you inform your doctor if you are already being treated with any of the following:
- Medicines to prevent blood clots or that decrease the number of platelets in the blood, such as acetylsalicylic acid, heparin, warfarin, clopidogrel, rivaroxaban, and dabigatran.
- Any medicine that may alter kidney function or that may damage the kidneys, such as sulfonamides (used as antibiotics), anilides (used to treat fever and pain), aldosterone antagonists (used as a diuretic), and natural and other opioid alkaloids (used to treat pain).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
Women of childbearing age
Tegsedi reduces the level of vitamin A in your body, which is essential for normal fetal development during pregnancy. It is not known whether vitamin A supplementation can compensate for the risk of vitamin A deficiency that may affect your unborn child (see "Warnings and precautions" above). If you are a woman of childbearing age, you must use effective contraceptive methods, and pregnancy should be ruled out before starting treatment with Tegsedi.
Pregnancy
You should not use Tegsedi if you are pregnant, unless your doctor advises you to do so.
Breastfeeding
Inotersen may pass into breast milk. The risk to the breastfed child cannot be ruled out. You should consult your doctor if you need to interrupt breastfeeding or discontinue Tegsedi treatment.
Driving and using machines
The use of Tegsedi has not been shown to affect the ability to drive or use machines.
Tegsedi contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per 1.5 ml, which is essentially "sodium-free".
3. How to use Tegsedi
Follow your doctor's instructions for administering this medication exactly. If in doubt, consult your doctor or pharmacist again.
The recommended dose of Tegsedi is a dose of 284 mg of inotersen.
Doses should be administered once a week. All subsequent doses should be injected once a week, on the same day of each week.
Route and method of administration
Tegsedi is for subcutaneous injection only.
Instructions for use
Before using the pre-filled syringe, your doctor will show you or your caregiver how to use it correctly. If you or your caregiver have any doubts, ask your doctor.
Read the instructions for use before starting to use the pre-filled syringe and each time you have the prescription repeated, as there may be new information.
Guide to parts
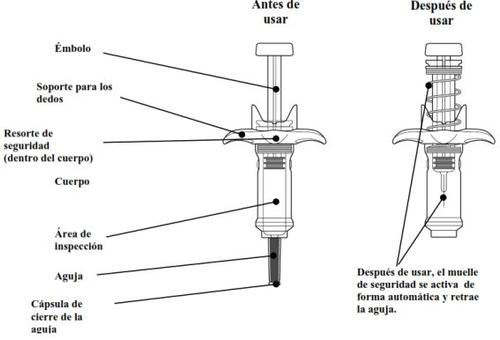
Each pre-filled syringe contains a dose and is for single use only.
WARNINGS | |
Do notremove the needle cap until you have reached Step 6of these instructions and are ready to inject Tegsedi. Do notshare the syringe with another person or reuse the syringe. Do notuse the pre-filled syringe if it falls on a hard surface or is damaged. Do notfreeze the pre-filled syringe. If any of the above occurs, discard the pre-filled syringe in a puncture-proof container and use a new pre-filled syringe. | |
PREPARATION | |
| |
Do notinject the medication until you have gathered the listed items. | |
| |
Do not move the plunger. | |
| |
| Look at the inspection area to check that the solution is clear and colorless or pale yellow. It is normal to see bubbles in the solution. You do not need to do anything about it. Do notuse if the solution appears cloudy, discolored, or contains particles. If the solution appears cloudy, discolored, or contains particles, discard the pre-filled syringe in a puncture-proof container and use a new pre-filled syringe. |
| |
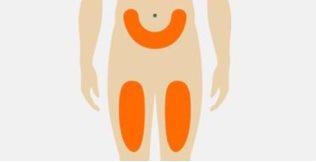
| Select the injection site on the abdomen (stomach) or the front of the thigh. The injection site may also be the outer area of the upper arm if Tegsedi is administered by a caregiver. Do notinject into an area within 3 cm of the navel. Do notinject into the same area each time. Do notinject if the skin is bruised, painful, red, or hard. Do notinject into tattoos, scars, or damaged skin. Do notinject through clothing. |
| |
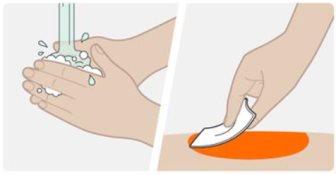
| Wash your hands with water and soap. Clean the injection site with an alcohol swab using a circular motion. Let the skin air dry. Do not touch the area again before injecting. |
INJECTION | |
| |
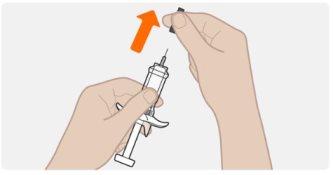
| Hold the pre-filled syringe by the body, with the needle pointing outward. Remove the needle cap by pulling it straight off. Do not twist it. You may see a drop of liquid at the end of the needle. This is normal. Keep your hands away from the plunger to avoid pushing it before you are ready to inject. Do notremove the needle cap until just before injecting. Do notpull the needle cap while holding the pre-filled syringe by the plunger. Always hold the syringe body. Do notlet the needle touch any surface. Do notremove any air bubbles from the pre-filled syringe. Do notput the needle cap back on the pre-filled syringe. |
| |
Hold the pre-filled syringe in one hand. Hold the skin around the injection site as your healthcare professional has instructed. You should gently pinch the skin at the injection site, or apply the injection without pinching the skin. Insert the needle slowly into the selected injection site at a 90° angle until it is fully inserted. Do nothold the pre-filled syringe by the plunger or push against the plunger to insert the needle. | |
| |
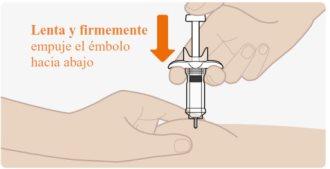
| Slowly and firmly push the plunger all the way down until the medication has been injected. Check that the needle remains fully inserted in the injection site while injecting the medication. It is important to push the plunger all the way down. You may hear a "click" as you push the plunger down. This is normal. Do notthink that the injection is finished. You may feel the plunger become stiff towards the end of the injection. You may need to press a little harder on the plunger to make sure you have pushed it as far as possible. Do notrelease the plunger. |
| |
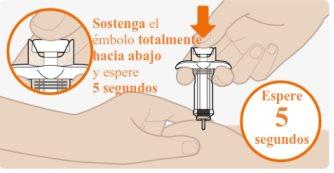
| Press firmly on the plunger at the end of the injection. Hold the plunger fully down and wait 5 seconds.If you release the plunger too quickly, you may lose some of the medication. The plunger will start to rise automatically, which means the plunger was pressed fully down. Press again if the plunger does not start to rise automatically. |
| |
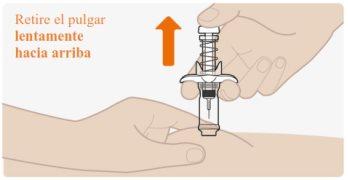
| Slowly release the plunger and let the safety spring automatically retract the plunger upward. The needle should now be safely retracted inside the pre-filled syringe, and the safety mechanism spring will be visible on the outside of the plunger. When the plunger stops, the injection is complete. If the plunger does not rise automatically when you release the pressure, it means the safety spring was not activated, and you should press the plunger again but with more force. Do notlift the plunger with your hand. Lift the entire pre-filled syringe straight up. Do nottry to put the needle cap back on the retracted needle. Do notrub the injection site. |
DISPOSAL AND CARE | |
Disposal of the used pre-filled syringe | |
| After use, immediately put the used pre-filled syringe in a puncture-proof container. Do not throw the pre-filled syringe in your household trash. |
If you use more Tegsedi than you should
Contact your doctor or pharmacist, or go immediately to the emergency department of a hospital, even if you do not have any symptoms.
If you forget to use Tegsedi
If you miss a dose of Tegsedi, the next dose will be given as soon as possible, unless the next scheduled dose is within 2 days, in which case you should skip the missed dose and give the next dose as scheduled.
Do nottake a double dose to make up for missed doses.
If you interrupt treatment with Tegsedi
Do not interrupt treatment with Tegsedi unless your doctor tells you to do so.
If you have any further questions about the use of this medication, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
If you experience any of the following side effects, stop using Tegsedi and contact your doctor immediately:
- Symptoms that may indicate glomerulonephritis (where your kidneys do not work properly), such as foam in the urine, pink or brown urine, blood in the urine, or urinating less than usual. Glomerulonephritis is a frequent side effect of inotersen (it may affect up to 1 in 10 people).
- Symptoms that may indicate thrombocytopenia (the blood does not clot due to low platelet levels in the blood), such as unexplained bruising or a rash of small red patches on the skin (called petechiae), bleeding from cuts in the skin that does not stop or oozes, nosebleeds or bleeding from the gums, blood in the urine or stool, or bleeding in the white part of the eyes. Low platelet levels in the blood are a very frequent side effect of inotersen (it may affect up to 1 in 10 people).
- Symptoms that may indicate liver damage, such as yellowing of the eyes or skin, or dark urine, possibly accompanied by itching of the skin, pain in the upper right part of the stomach (abdomen), loss of appetite, bleeding or bruising more easily than normal, or feeling tired.
Seek immediate attention if you experience stiffness in the neck or an unusual and severe headache, as these symptoms can be caused by a brain hemorrhage.
Other side effects
Very common(may affect more than 1 in 10 people)
- Decrease in red blood cells that can cause paleness of the skin and lead to weakness or difficulty breathing (anemia)
- Headache
- Vomiting or nausea (feeling sick) - Increased body temperature
- Feeling cold or chills
- Pain at the injection site, redness, itching, or bruising
- Swelling in the ankles, feet, or fingers (peripheral edema)
Common(may affect up to 1 in 10 people)
- Increased levels of white blood cells called eosinophils (eosinophilia)
- Decreased appetite
- Feeling dizzy or faint, especially when standing up (low blood pressure, hypotension)
- Bruising
- Accumulation of blood in the tissues, which can be seen as significant bruising (hematomas)
- Itching
- Rash
- Kidney damage that can cause impaired kidney function or kidney failure
- Changes in blood and urine test results (this may indicate liver or kidney damage)
- Flu-like symptoms, such as high temperature, pain, and chills
- Swelling or discoloration of the skin at the injection site
Uncommon(may affect up to 1 in 100 people)
- Allergic reaction
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Tegsedi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the carton, tray, and pre-filled syringe after EXP. The expiration date is the last day of the month indicated.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze.
Tegsedi can be stored without refrigeration for up to 6 weeks at a temperature below 30°C. If not refrigerated and not used within 6 weeks, the medicine should be discarded.
Store in the original packaging to protect it from light.
Do not use this medicine if you notice that the contents are cloudy or contain particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package contents and additional information
Composition of Tegsedi
- The active substance is inotersen.
Each ml contains 189 mg of inotersen (as inotersen sodium). Each pre-filled syringe contains 284 mg of inotersen (as inotersen sodium) in 1.5 ml of solution.
- The other ingredients are water for injections, sodium hydroxide, and hydrochloric acid (see "Tegsedi contains sodium" in section 2).
Appearance and package contents
Tegsedi is a clear, colorless to pale yellow injectable solution in a pre-filled syringe.
Tegsedi is available in pack sizes of 1 or 4 pre-filled syringes.
Not all pack sizes may be marketed.
Marketing authorization holder
Akcea Therapeutics Ireland Ltd
St. James House,
72 Adelaide Road, Dublin 2
D02 Y017, Ireland
Manufacturer
ABF Pharmaceutical Services GmbH
Brunnerstraße 63/18-191230 Vienna
Austria
Date of last revision of this leaflet: 11/2023
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TEGSEDI 284 mg Injectable Solution in Pre-filled SyringeDosage form: INJECTABLE, 25 mgActive substance: vutrisiranManufacturer: Alnylam Netherlands B.V.Prescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 300 mg/mlActive substance: sodium oxybateManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 50 mgActive substance: riluzoleManufacturer: Zambon S.P.A.Prescription required
Online doctors for TEGSEDI 284 mg Injectable Solution in Pre-filled Syringe
Discuss questions about TEGSEDI 284 mg Injectable Solution in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions