
TALIDOMIDA ACCORD 50 MG CAPSULAS DURAS EFG

Cómo usar TALIDOMIDA ACCORD 50 MG CAPSULAS DURAS EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Talidomida Accord 50 mg cápsulas duras EFG
ADVERTENCIA
Talidomida provoca defectos congénitos y muerte del feto. No tome talidomida si está o pudiera quedarse embarazada. Tiene que seguir los consejos sobre anticoncepción de su médico
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Talidomida Accord y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Talidomida Accord
- Cómo tomar Talidomida Accord
- Posibles efectos adversos
- Conservación de Talidomida Accord
- Contenido del envase e información adicional
1. Qué es Talidomida Accord y para qué se utiliza
Qué es Talidomida Accord
Talidomida Accord contiene un principio activo denominado talidomida, que pertenece a un grupo de fármacos que afectan al modo en que funciona su sistema inmunitario.
Para qué se utiliza Talidomida Accord
Talidomida se utiliza con otros dos medicamentos llamados “melfalán” y “prednisona” para tratar a adultos con un tipo de cáncer llamado mieloma múltiple. Se utiliza en personas de 65 años o más que han sido recientemente diagnosticadas y que no han utilizado antes ningún medicamento para el mieloma múltiple, o en personas de menos de 65 años que no pueden ser tratadas con quimioterapia a altas dosis, ya que puede ser difícil de asimilar para el organismo.
Qué es el mieloma múltiple
El mieloma múltiple es un tipo de cáncer que afecta a un tipo de glóbulos blancos, llamados células plasmáticas. Estas células se producen en la médula ósea y se dividen sin control. Esto puede dañar los huesos y los riñones. El mieloma múltiple por lo general no tiene cura. Sin embargo, los signos y síntomas pueden disminuir en gran medida o desaparecer durante un periodo de tiempo. A esto se le llama “remisión”.
Cómo actúa Talidomida Accord
Talidomida actúa ayudando al sistema inmunitario del organismo y atacando directamente al cáncer. Actúa de diferentes maneras:
- Frena el desarrollo de las células cancerosas.
- Frena el crecimiento de vasos sanguíneos en el cáncer.
- Estimula parte del sistema inmunitario para que ataque a las células cancerosas.
.
2. Qué necesita saber antes de empezar a tomar Talidomida Accord
Debe recibir instrucciones específicas de su médico, particularmente sobre los efectos de talidomida en el feto (que se explican en el Programa de Prevención de Embarazo de Talidomida Accord).
Debe recibir un folleto informativo para pacientes de su médico. Debe leer detenidamente y seguir sus instrucciones.
Si no entiende completamente estas instrucciones, pídale a su médico que se las explique de nuevo antes de tomar talidomida. Puede encontrar más información en esta sección en los apartados “Advertencias y precauciones” y “Embarazo y lactancia”.
No tome Talidomida Accord
- Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, ya que talidomida provoca defectos congénitos y muerte del feto.
- Si tiene posibilidad de quedarse embarazada a menos que sea capaz de seguir o cumplir las medidas anticonceptivas exigidas para evitar quedarse embarazada (ver sección 2 “Advertencias y precauciones” y “Embarazo y lactancia”).
- Si tiene posibilidad de quedarse embarazada, su médico registrará con cada receta, que se han tomado las medidas necesarias y le proporcionará esta confirmación.
- Si es alérgico a talidomida o a alguno de los demás componentes de este medicamento incluidos en la sección 6 “Contenido del envase e información adicional”.
No tome talidomida si cualquiera de los puntos anteriores le aplica. Si no está seguro/a, consulte a su médico o farmacéutico antes de empezar a tomar talidomida
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a tomar este medicamento en los siguientes casos:
Para las mujeresque toman talidomida
Antes de iniciar el tratamiento, debe consultar con su médico si existe la posibilidad de que pueda quedarse embarazada, aunque crea que esto es poco probable. Incluso si no tiene sangrado menstrual tras un tratamiento para el cáncer, puede quedarse embarazada.
Si tiene posibilidades de quedarse embarazada:
- Su médico se asegurará de que le hagan las pruebas de embarazo
- Antes del tratamiento.
- Cada 4 semanas durante el tratamiento
- 4 semanas después de finalizar el tratamiento
- Debe usar un método eficaz de anticoncepción:
- Durante al menos 4 semanas antes de empezar el tratamiento.
- Durante el tratamiento.
- Hasta al menos las 4 semanas siguientes a la finalización del tratamiento.
Su médico le indicará qué método anticonceptivo debe usar.
Si tiene probabilidades de quedarse embarazada, su médico registrará con cada receta que se han tomado las medidas necesarias, descritas anteriormente.
Para los hombresque toman talidomida
Talidomida pasa al semen. Por lo tanto, no mantenga relaciones sexuales sin protección, incluso si se ha sometido a una vasectomía.
- Debe evitar el embarazo y cualquier exposición durante el embarazo. Use siempre un preservativo:
- Durante el tratamiento.
- Durante al menos 7 días después de finalizar el tratamiento.
- No debe donar semen:
- Durante el tratamiento.
- Durante al menos 7 días después de finalizar el tratamiento.
Para todos los pacientes
Antes de tomar talidomida consulte a su médico si:
- No entiende las indicaciones sobre anticoncepción que le ha dado su médico o no cree ser capaz de seguirlos.
- Ha tenido un ataque al corazón, alguna vez en el pasado ha tenido un coágulo de sangre, o si fuma, o tiene la tensión arterial alta o los niveles de colesterol altos. Durante el tratamiento con talidomida, tiene un mayor riesgo de desarrollar coágulos de sangre en las venas y en las arterias (ver también sección 4 “posibles efectos secundarios”);
- Ha tenido o tiene neuropatía, es decir, daños en los nervios que provocan hormigueo, coordinación anormal o dolor en las manos o pies (ver también sección 4 “posibles efectos secundarios”).
- Ha tenido o tiene la frecuencia cardiaca lenta (puede ser un síntoma de bradicardia).
- Tiene la tensión arterial alta en las arterias que salen de los pulmones (ver también sección 4 “posibles efectos secundarios”).
- Sufre una disminución del número de leucocitos (neutropenia) acompañada de fiebre e infección.
- Sufre una disminución del número de plaquetas. Será más propenso a sufrir hemorragia y moratones.
- Tiene o ha tenido alguna lesión en el hígado (trastornos hepáticos), tales como resultados anormales en pruebas hepáticas.
- Ha tenido erupciones cutáneas graves que pueden empezar como una erupción cutánea en un área, pero que se extiende con ampollas en la piel y la mucosa (síndrome de Stevens-Johnson y necrólisis epidérmica tóxica). Es posible que tenga fiebre al mismo tiempo.
- Ha tenido una reacción alérgica mientras tomaba talidomida, como erupción, picor, hinchazón, mareos o problemas respiratorios.
- Ha tenido somnolencia.
- Ha tenido fiebre, escalofríos y temblores intensos, y posiblemente se han complicado con tensión arterial baja y confusión (pueden ser síntomas de infecciones graves).
- Tiene o ha tenido alguna vez una infección vírica, en concreto varicela zóster, infección de la hepatitis B o VIH. En caso de duda, consulte a su médico. El tratamiento con talidomida puede hacer que el virus se active de nuevo en los pacientes portadores del mismo, dando lugar a la reaparición de la infección. Su médico debe comprobar si alguna vez ha tenido una infección de la hepatitis B;
- Tiene problemas renales o hepáticos (ver también sección 4 “posibles efectos secundarios”);
Su médico puede comprobar si tiene una cantidad total de tumores por todo el cuerpo elevada, incluyendo la médula ósea. Esto podría dar lugar a una enfermedad en la que los tumores se descomponen y producen niveles anómalos de químicos en la sangre que pueden producir un fallo renal (esta enfermedad se llama síndrome de lisis tumoral) (ver también sección 4 “Posibles efectos secundarios”).
Su médico debe evaluar si presenta otros tipos de neoplasias hematológicas malignas (llamadas leucemia mielógena aguda y síndromes mielodisplásicos) durante su tratamiento con talidomida (ver también sección 4 “Posibles efectos secundarios”).
No debe donar sangre durante el tratamiento con talidomida , ni en al menos 7 días después de la finalización del tratamiento.
Si no está seguro/a de si alguno de los casos anteriores le aplica, consulte a su médico antes de empezar a tomar talidomida.
Niños y adolescentes
No se recomienda el uso de talidomida en niños y adolescentes menores de 18 años.
Uso de Talidomida Accord con otros medicamentos
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente cualquier otro medicamento, incluso los adquiridos sin receta y los preparados a base de plantas.
Asegúrese de advertir a su médico si está tomando algún medicamento que:
- Cause somnolencia, ya que talidomida podría incrementar ese efecto. Entre estos medicamentos se encuentran los sedantes (como ansiolíticos, hipnóticos, antipsicóticos, antihistamínicos H1, derivados del opio y barbitúricos).
- Ralentice la frecuencia cardiaca (induzca bradicardia, como las anticolinesterasas y los betabloqueantes).
- Se utilice para problemas y complicaciones del corazón (como la digoxina) o para fluidificar la sangre (como la warfarina).
- Esté asociado a neuropatía, tales como otros tratamientos para el cáncer.
- Se utilice para anticoncepción.
Toma de Talidomida Accord con alimentos, bebidas y alcohol
No beba alcohol si está tomando talidomida. El motivo es que el alcohol le dará sueño y talidomida puede darle aún más.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Embarazo
Talidomida provoca defectos de nacimiento graves y la muerte del feto.
- El hecho de que una mujer embarazada tome incluso una dosis tan pequeña como una cápsula puede hacer que el bebé nazca con defectos congénitos graves.
- Estos defectos pueden incluir brazos o piernas más cortos, deformación en manos o pies, problemas en los ojos, en los oídos o en los órganos internos.
Si está embarazada no debe tomar talidomida . Además, no debe quedarse embarazada si está tomando talidomida .
Las mujeres que puedan quedarse embarazadas deben usar un método anticonceptivo eficaz (consulte la sección 2, “Qué necesita saber antes de empezar a tomar Talidomida Accord”
Debe interrumpir el tratamiento e informar inmediatamente a su médico si:
- No tiene un período o cree que no ha tenido un período, en caso de hemorragia menstrual no habitual o si sospecha que puede estar embarazada.
- Mantiene relaciones heterosexuales sin usar un método anticonceptivo eficaz.
Si se queda embarazada durante el tratamiento con talidomida, tiene que interrumpir el tratamiento e informar a su médico inmediatamente.
Los hombres que tomen talidomida y tengan una pareja que pueda quedarse embarazada deben leer la sección 2 “Qué necesita saber antes de empezar a tomar Talidomida Accord”. Si su pareja se queda embarazada mientras usted toma talidomida, debe informar a su médico inmediatamente.
Lactancia
No debe dar el pecho cuando tome talidomida , ya que no se sabe si talidomida pasa a la leche materna humana.
Conducción y uso de máquinas
No conduzca ni use herramientas ni máquinas si sufre efectos adversos como mareos, cansancio, somnolencia o visión borrosa.
Este medicamento contiene Isomalta.
Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
Este medicamento contiene menos de 23 mg de sodio (1mmol) por cápsula esto es, esencialmente “exento de sodio”.
3. Cómo tomar Talidomida Accord
Siga exactamente las instrucciones de administración de talidomida indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Cuánto tomar
La dosis recomendada es de 200 mg (4 cápsulas de 50 mg) al día en adultos de 75 años o menos o de 100 mg (2 cápsulas de 50 mg) al día en adultos mayores de 75 años. Sin embargo, su médico elegirá la dosis más adecuada en su caso, hará un seguimiento de su progreso y podrá ajustar la dosis. Su médico le indicará cómo debe tomar talidomida y durante cuánto tiempo tendrá que tomarlo (ver sección 2, “Qué necesita saber antes de empezar a tomar Talidomida Accord”).
Talidomida se toma cada día en ciclos de tratamiento, cada uno de los cuales dura 6 semanas, en combinación con melfalán y prednisona, que se toman los días 1 a 4 de cada ciclo de 6 semanas.
Cómo tomar estemedicamento
- No rompa, abra ni mastique las cápsulas. Si los polvos de una cápsula rota de talidomida entran en contacto con la piel, lave la piel de forma inmediata y cuidadosa con agua y jabón.
- Tome este medicamento por vía oral.
- Tráguese las cápsulas enteras con un vaso lleno de agua.
- No las machaque ni las mastique.
- Tome las cápsulas como dosis única antes de irse a dormir. Esto hará que sienta menos somnolencia en otros momentos.
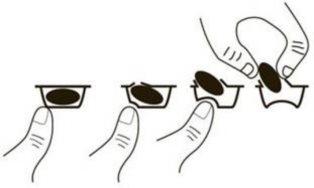
Para sacar la cápsula del blíster, presione solo un extremo de la cápsula para que salga a través de la lámina. No presione en el centro de la cápsula ya que podría romperla.
Si toma más Talidomida Accord del que debe
Si toma una cantidad de talidomida mayor que la indicada, consulte a un médico o vaya directamente a un hospital. Si es posible, lleve consigo el envase del medicamento y este prospecto.En caso de sobredosis o ingestión accidental consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó tomar Talidomida Accord
Si se olvida de tomar talidomida a su hora habitual y
- Han transcurrido menos de 12 horas: tome las cápsulas inmediatamente.
- Han transcurrido más de 12 horas: no tome las cápsulas. Tome las siguientes cápsulas a la hora habitual al día siguiente.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Este medicamento puede causar los siguientes efectos adversos:
Deje de tomar talidomida y consulte inmediatamente a un médico si nota los siguientes efectos adversos graves – podría necesitar tratamiento médico urgente:
- Reacciones cutáneas graves, como erupciones cutáneas, que es un efecto adverso común y ampollas en la piel y mucosa (síndrome de Stevens-Johnson y necrólisis epidérmica tóxica, que son efectos adversos raros). Puede tener temperatura alta (fiebre) al mismo tiempo.
Consulte inmediatamente a su médico si nota cualquiera de los siguientes efectos adversos graves:
- Entumecimiento, hormigueo, coordinación anormal o dolor en las manos y pies.
Esto puede deberse a daños en los nervios (neuropatía periférica), que es un efecto adverso muy frecuente. Puede llegar a ser muy grave, doloroso e incapacitante. Si presenta estos síntomas, consulte a su médico inmediatamente, que puede reducir la dosis o interrumpir el tratamiento. Este efecto adverso generalmente se presenta después de tomar este medicamento durante algunos meses, pero puede suceder antes. También puede suceder algún tiempo después de la finalización del tratamiento. Puede que no desaparezca o que lo haga lentamente.
- Dolor repentino en el pecho o dificultad para respirar.
Puede deberse a coágulos de sangre en las arterias que van hasta los pulmones (embolia pulmonar), que es un efecto adverso frecuente. Puede aparecer durante el tratamiento o una vez finalizado.
- Dolor o inflamación en las piernas, sobre todo en la parte inferior o en las pantorrillas.
Puede deberse a coágulos de sangre en las venas de las piernas (trombosis venosa profunda), que es un efecto adverso frecuente. Puede aparecer durante el tratamiento o una vez finalizado el tratamiento.
- Dolor de pecho que se extiende a brazos, cuello, mandíbula, espalda o estómago, sudoración y falta de aliento, náuseas o vómitos.
Pueden ser síntomas de un ataque al corazón/infarto de miocardio (que se puede deber a coágulos sanguíneos en las arterias del corazón).
- Dificultad temporal para ver o hablar.
Pueden ser síntomas de un infarto cerebral (ictus) (que se puede deber a un coágulo sanguíneo en una arteria del cerebro).
- Fiebre, escalofríos, dolor de garganta, tos, úlceras bucales o cualquier otro síntoma de infección.
- Hemorragia o moratones en ausencia de lesión.
Otros efectos adversos incluyen:
Es importante señalar que un número pequeño de pacientes con mieloma múltiple puede desarrollar otros tipos de cáncer, especialmente neoplasias hematológicas malignas, y es posible que este riesgo aumente con el tratamiento con talidomida; por lo tanto, su médico debe evaluar cuidadosamente los beneficios y los riesgos al recetarle talidomida.
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- Estreñimiento.
- Mareos.
- Somnolencia, cansancio.
- Temblores.
- Disminución de las sensaciones o sensaciones anormales (disestesia).
- Inflamación de manos y pies.
- Recuentos bajos de células sanguíneas, lo que podría significar una mayor probabilidad de desarrollar infecciones. Su médico puede controlar los recuentos de sus células sanguíneas durante el tratamiento con talidomida.
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- Indigestión, náuseas, vómitos, boca seca.
- Erupción, piel seca.
- Disminución del número de leucocitos (neutropenia) acompañada de fiebre e infección.
- Disminución del número de glóbulos rojos y leucocitos, y de plaquetas al mismo tiempo (pancitopenia).
- Debilidad, desmayo o inestabilidad, falta de energía o fuerza, tensión arterial baja.
- Fiebre, malestar general.
- Convulsiones.
- Sensación de que la cabeza le da vueltas, lo que hace que resulte difícil estar de pie y moverse con normalidad.
- Visión borrosa.
- Neumonía, enfermedad pulmonar.
- Ritmo cardíaco lento, insuficiencia cardiaca.
- Depresión, confusión, cambios en el humor, ansiedad.
- Audición disminuida o sordera.
- Enfermedad renal (fallo renal).
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- Inflamación e hinchazón de los tubos pulmonares (bronquitis).
- Inflamación de la pared del estómago.
- Perforación del intestino grueso (colon), lo que puede causar una infección.
- Obstrucción intestinal.
- Disminución de la presión arterial en posición de pie, que puede provocar desfallecimiento.
- Latido cardiaco irregular (bloqueo cardiaco o fibrilación auricular), sensación de pérdida del conocimiento o desfallecimiento.
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- Tiroides hipoactiva (hipotiroidismo).
- Obstrucción intestinal.
- Disfunción sexual, por ejemplo, impotencia.
- Infección grave de la sangre (septicemia) acompañada de fiebre, escalofríos y temblores graves, y posiblemente complicada por tensión arterial baja y confusión (shock séptico).
- Síndrome de lisis tumoral – complicaciones metabólicas que pueden producirse durante el tratamiento del cáncer y a veces incluso sin tratamiento. Estas complicaciones se deben a los productos de descomposición de las células cancerosas que mueren y pueden incluir lo siguiente: cambios en la bioquímica sanguínea; niveles altos de potasio, fósforo, ácido úrico y niveles bajos de calcio, que como consecuencia, producen cambios en la función renal, latido cardiaco, convulsiones y, a veces, la muerte.
- Reacciones alérgicas tales como exantema pruriginoso localizado o generalizado y angioedema (tipos de reacciones alérgicas que pueden manifestarse como urticaria, erupciones cutáneas, inflamación de los ojos, la boca o la cara, dificultad para respirar o picor).
- Lesión hepática (trastorno hepático) incluidos resultados anómalos en las pruebas de la función hepática.
- Hemorragia en el estómago o los intestinos (hemorragia gastrointestinal).
- Empeoramiento de los síntomas de la enfermedad de Parkinson (tales como temblores, depresión o confusión).
- Dolor en la parte superior del abdomen y/o espalda, que puede ser intenso y durar varios días, posiblemente acompañado de náuseas, vómitos, fiebre y pulso rápido – estos síntomas pueden deberse a la inflamación del páncreas (pancreatitis).
- Aumento de la presión arterial en los vasos sanguíneos que suministran la sangre a los pulmones, lo que puede dar lugar a dificultad respiratoria, cansancio, mareo, dolor de pecho, latido cardiaco más rápido o hinchazón de piernas o tobillos (hipertensión pulmonar).
- Infecciones víricas, incluido el herpes zóster (también llamado «culebrilla», una enfermedad vírica que produce un doloroso sarpullido con ampollas) y recurrencia de la infección de la hepatitis B (que puede producir que la piel y los ojos tomen un color amarillo, orina de color marrón oscuro, dolor de estómago en el lado derecho, fiebre, náuseas o vómitos).
- Una enfermedad del cerebro con síntomas como cambios en la visión, cefalea, convulsiones y confusión, con o sin tensión arterial alta (síndrome de encefalopatía posterior reversible o PRES).
- Una enfermedad que afecta a la piel causada por la inflamación de pequeños vasos sanguíneos, acompañada de dolor en las articulaciones y fiebre (vasculitis leucocitoclástica).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Talidomida Accord
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y en el blíster, después de CAD. La fecha de caducidad es el último día del mes que se indica.
No utilice este medicamento si observa deterioro o signos de manipulación del cierre de garantía.
No conservar a temperatura superior a 30°C
Al final de su tratamiento debe devolver todas las cápsulas no utilizadas al farmacéutico o al médico, para evitar el uso indebido.
6. Contenido del envase e información adicional
Composición de Talidomida Accord:
- El principio activo es talidomida. Cada cápsula contiene 50 mg de talidomida.
- Los demás excipientes son isomalta (E953), croscarmelosa sódica y fumarato de estearilo y sodio. La cubierta de la cápsula contiene gelatina y dióxido de titanio (E171).
Aspecto del producto y contenido del envase
Las cápsulas duras de Talidomida Accord son blancas opacas del número 4. Las cápsulas están rellenas de un polvo blanco.
Blíster de PVC/PCTFE/aluminio que contiene 14 cápsulas. Tamaño del envase: Díptico con 28 cápsulas (2 blísteres)
Blíster unidosis de PVC/PCTFE/aluminio que contiene 7 cápsulas. Tamaño del envase: 28 cápsulas (4 blísteres) en estuches.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Accord Healthcare, S.L.U.
World Trade Center,
Moll de Barcelona, s/n,
Edifici Est, 6ª planta,
08039 Barcelona
España
Responsable de la fabricación
Ardena Pamplona, S.L.
Pol Mocholi, Calle Noáin 1
31110 Noáin, Navarra
España
O
Medichem S.A.
Narcis Monturiol, 41A
08970 Sant Joan Despí - Barcelona.
España
O
Bluepharma – Indústria Farmacêutica, S.A.
Eiras, Rua Adriano Lucas
Coimbra, 3020-430
Portugal
O
Bluepharma – Indústria Farmacêutica, S.A.
- Martinho do Bispo
Coimbra, 3045-016
Portugal
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Chipre Thalidomide Accord 50mg σκληρ? καψ?κια
Dinamarca Thalidomide Accord
Eslovenia Talidomid Accord 50mg trde kapsule
España Talidomida Accord 50 mg cápsulas duras EFG
Francia Thalidomide Accord 50mg gélule
Hungría Thalidomide Accord 50mg kemény kapszula
Italia Talidomide Accord
Malta Thalidomide Accord 50mg hard capsules
Polonia Thalidomide Accord
Portugal Thalidomide Accord 50mg cápsulas
Rumanía Talidomida Accord 50 mg capsule
Fecha de la última revisión de este prospecto: 02/2022
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios: http://www.aemps.gob.es.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TALIDOMIDA ACCORD 50 MG CAPSULAS DURAS EFGForma farmacéutica: CAPSULA, 50 mgPrincipio activo: TalidomidaFabricante: Bristol-Myers Squibb Pharma EeigRequiere recetaForma farmacéutica: INYECTABLE, 10 mg/ 1 mlPrincipio activo: MetotrexatoFabricante: Ebewe Pharma Ges.M.B.H. Nfg.KgRequiere recetaForma farmacéutica: INYECTABLE, 15 mgPrincipio activo: MetotrexatoFabricante: Ebewe Pharma Ges.M.B.H. Nfg.KgRequiere receta
Médicos online para TALIDOMIDA ACCORD 50 MG CAPSULAS DURAS EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TALIDOMIDA ACCORD 50 MG CAPSULAS DURAS EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






