
SEMGLEE 100 UNIDADES/ML SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar SEMGLEE 100 UNIDADES/ML SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Semglee 100 unidades/ml solución inyectable en pluma precargada
insulina glargina
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Semglee y para qué se utiliza
- Qué necesita saber antes de empezar a usar Semglee
- Cómo usar Semglee
- Posibles efectos adversos
- Conservación de Semglee
- Contenido del envase e información adicional
1. Qué es Semglee y para qué se utiliza
Semglee contiene insulina glargina. Esta es una insulina modificada, muy similar a la insulina humana.
Semglee se utiliza en el tratamiento de la diabetes mellitus en pacientes adultos, adolescentes y niños a partir de los 2 años.
La diabetes mellitus es una enfermedad en la que su organismo no produce suficiente insulina para controlar el nivel de azúcar en la sangre. La insulina glargina tiene una acción prolongada y constante de reducción de azúcar en sangre.
2. Qué necesita saber antes de empezar a usar Semglee
No use Semglee
- si es alérgico a la insulina glargina o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Semglee en pluma precargada solo es adecuado para inyecciones subcutáneas. Consulte a su médico si es necesario inyectarle su insulina por otro método.
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Semglee.
Respete estrictamente las instrucciones sobre posología, control (pruebas de sangre y orina), dieta y actividad física (trabajo físico y ejercicio), y técnica de inyección que ha establecido con su médico.
Si su azúcar en sangre es demasiado bajo (hipoglucemia), siga la guía sobre hipoglucemia (ver el recuadro que aparece al final de este prospecto).
Viajes
Antes de viajar, consulte con su médico. Tal vez tenga que consultar sobre:
- la disponibilidad de su insulina en el país que va a visitar,
- reservas de insulina, agujas, etc.,
- el almacenamiento correcto de la insulina durante el viaje,
- el horario de las comidas y de la administración de insulina durante el viaje,
- los posibles efectos del traslado a zonas con diferencias horarias,
- los posibles nuevos riesgos para la salud en los países que va a visitar,
- qué debe hacer en situaciones de urgencia cuando se encuentre mal o se ponga enfermo.
Enfermedades y lesiones
El manejo de su diabetes puede necesitar un cuidado especial en las siguientes situaciones (por ejemplo, ajuste de la dosis de insulina, análisis de sangre y orina):
- Si está enfermo o sufre una lesión importante, puede aumentar su nivel de azúcar en sangre (hiperglucemia).
- Si no come lo suficiente, su nivel de azúcar en sangre puede bajar demasiado (hipoglucemia).
En la mayoría de los casos necesitará un médico. Asegúrese de consultar inmediatamente a un médico.
Si padece usted diabetes tipo 1 (diabetes mellitus dependiente de insulina), no deje de administrarse su insulina y de seguir tomando suficientes hidratos de carbono. Informe siempre a las personas que se ocupan de su cuidado o tratamiento de que necesita insulina.
El tratamiento con insulina puede causar que su cuerpo produzca anticuerpos a la insulina (sustancias que actúan frente a la insulina). Sin embargo, solamente en muy raras ocasiones, necesitará cambiar su dosis de insulina.
Algunos pacientes con diabetes mellitus tipo 2 de larga duración y enfermedad cardíaca o accidente cerebrovascular previo que fueron tratados con pioglitazona (medicamento antidiabético oral utilizado para el tratamiento de la diabetes mellitus tipo 2) e insulina sufrieron desarrollo de insuficiencia cardíaca. Informe a su médico lo antes posible si sufre signos de insuficiencia cardíaca como falta de aliento poco corriente o aumento rápido de peso o hinchazón localizada (edema).
Niños
No hay experiencia con el uso de Semglee en niños menores de 2 años.
Uso de Semglee con otros medicamentos
Algunos medicamentos producen cambios en los niveles de azúcar en sangre (aumento, descenso o ambos, dependiendo de la situación). En cada caso, puede ser necesario ajustar su dosis de insulina para evitar niveles de azúcar en sangre demasiado bajos o demasiado altos. Hay que tener cuidado cuando empiece a tomar otro medicamento y también cuando deje de tomarlo.
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente, o podría tener que tomar cualquier otro medicamento. Pregunte a su médico, antes de tomar un medicamento, si éste puede afectar a su nivel de azúcar en sangre, y qué medidas debe adoptar en su caso.
Entre los medicamentos que pueden provocar un descenso de su nivel de azúcar en sangre (hipoglucemia) se incluyen:
- todos los demás medicamentos para tratar la diabetes,
- los inhibidores de la enzima conversora de la angiotensina (ECA) (utilizados para tratar ciertas enfermedades del corazón o el aumento de la tensión arterial),
- la disopiramida (utilizada para tratar ciertas enfermedades del corazón),
- la fluoxetina (utilizada para tratar la depresión),
- los fibratos (utilizados para reducir los niveles elevados de lípidos en sangre),
- los inhibidores de la monoaminoxidasa (MAO) (utilizados para tratar la depresión),
- la pentoxifilina, el propoxifeno, los salicilatos (como el ácido acetilsalicílico, utilizado para aliviar el dolor y bajar la fiebre),
- los antibióticos del grupo de las sulfamidas.
Entre los medicamentos que pueden provocar un aumento de su nivel de azúcar en sangre (hiperglucemia) se incluyen:
- los corticosteroides (como la “cortisona”, utilizada para tratar la inflamación),
- el danazol (medicamento que actúa sobre la ovulación),
- el diazóxido (utilizado para tratar la tensión arterial alta),
- los diuréticos (utilizados para tratar la tensión arterial alta o el exceso de retención de líquidos),
- el glucagón (hormona pancreática utilizada para tratar la hipoglucemia grave),
- la isoniazida (utilizada para tratar la tuberculosis),
- los estrógenos y progestágenos (como en la píldora anticonceptiva utilizada para el control de la natalidad),
- los derivados de la fenotiazina (utilizados para tratar las enfermedades psiquiátricas),
- la somatotropina (hormona del crecimiento),
- los medicamentos simpaticomiméticos (como la epinefrina [adrenalina] o el salbutamol, la terbutalina para tratar el asma),
- las hormonas tiroideas (utilizadas para tratar el mal funcionamiento de la glándula tiroidea),
- medicamentos antipsicóticos atípicos (como clozapina, olanzapina),
- inhibidores de la proteasa (utilizados para tratar el VIH).
Su nivel de azúcar en la sangre puede subir o bien bajar si toma:
- betabloqueantes (utilizados para tratar la tensión arterial alta),
- clonidina (utilizada para tratar la tensión arterial alta),
- sales de litio (utilizadas para tratar las enfermedades psiquiátricas).
La pentamidina (utilizada para tratar algunas infecciones causadas por parásitos) puede causar una hipoglucemia, que algunas veces puede ir seguida de una hiperglucemia.
Los betabloqueantes, al igual que otros medicamentos simpaticolíticos (como clonidina, guanetidina y reserpina) pueden atenuar o suprimir por completo los primeros síntomas de aviso que podrían ayudarle a reconocer una hipoglucemia.
Si no está usted seguro de si está tomando alguno de estos medicamentos, pregunte a su médico o farmacéutico.
Uso de Semglee con alcohol
Sus niveles de azúcar en sangre pueden subir o bajar si bebe alcohol.
Embarazo y lactancia
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Informe a su médico si está planeando quedarse embarazada o si ya lo está. Su dosis de insulina puede requerir cambios durante el embarazo y tras el parto. Un control especialmente cuidadoso de su diabetes, y la prevención de la hipoglucemia, son importantes para la salud de su bebé.
Si está en el período de lactancia, consulte a su médico puesto que puede necesitar ajustes en su dosis de insulina y en su dieta.
Conducción y uso de máquinas
Su capacidad de concentración o de reacción puede verse reducida si:
- tiene hipoglucemia (niveles bajos de azúcar en sangre),
- tiene hiperglucemia (niveles altos de azúcar en sangre),
- tiene problemas de visión.
Esté atento a este posible problema, considerando todas las situaciones que pueden ser causa de riesgo para usted o para otros (como conducir un vehículo o utilizar máquinas). Debe pedir a su médico que le aconseje sobre la capacidad para conducir si:
- tiene frecuentes episodios de hipoglucemia,
- han disminuido o no aparecen los primeros síntomas de aviso que pueden ayudarle a reconocer una hipoglucemia.
Semglee contiene sodio
Este medicamento contiene menos de 1 mmol (23 mg) de sodio por dosis, esto es, esencialmente “exento de sodio”.
3. Cómo usar Semglee
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, pregunte a su médico o farmacéutico.
Aunque Semglee contiene el mismo principio activo que la insulina glargina 300 unidades/ml, estos medicamentos no son intercambiables. El cambio de un tratamiento con insulina a otro, necesita de prescripción médica, supervisión médica y control de la glucosa en sangre. Para más información, consulte con su médico.
Dosis
En función de su estilo de vida y los resultados de sus controles de azúcar (glucosa) en sangre y su anterior tratamiento con insulina, su médico:
- determinará la dosis de Semglee que necesita cada día y a qué hora,
- le indicará cuándo debe analizar su nivel de azúcar en sangre, y si necesita llevar a cabo análisis de orina,
- le indicará cuándo puede necesitar inyectarse una dosis más alta o más baja de Semglee.
Semglee es una insulina de acción larga. Su médico le puede indicar que la use en combinación con una insulina de acción corta o con comprimidos para tratar la elevación de los niveles de azúcar en sangre.
Muchos factores pueden influir en su nivel de azúcar en sangre. Debe conocer estos factores ya que así podrá reaccionar correctamente ante cambios de su nivel de azúcar en la sangre y prevenir que suba o baje demasiado. Para más información, ver el recuadro que aparece al final del prospecto.
Uso en niños y adolescentes
Semglee puede utilizarse en adolescentes y niños de 2 años y mayores. Utilice este medicamento tal y como le ha dicho su médico.
Frecuencia de administración
Necesita una inyección de Semglee cada día, siempre a la misma hora.
La pluma Semglee administra insulina en incrementos de 1 unidad hasta un máximo de dosis única de 80 unidades.
Forma de administración
Semglee se inyecta bajo la piel. NO se inyecte Semglee en una vena, porque esto cambiará su acción y puede provocar una hipoglucemia.
Su médico le mostrará en qué área de la piel debe usted inyectarse Semglee. Con cada inyección, debe cambiar el lugar de la punción dentro del área concreta de la piel que esté usando.
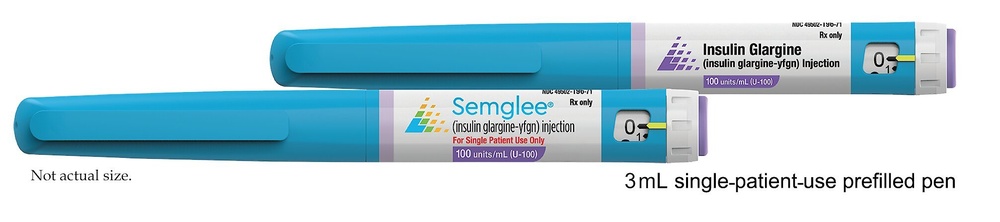
Cómo manejar la pluma Semglee
Semglee en pluma precargada solo es adecuado para inyecciones subcutáneas. Consulte a su médico si es necesario inyectar su insulina por otro método.
Lea cuidadosamente las “Instrucciones de Uso” incluidas en este prospecto. Usted debe utilizar la pluma tal y como se describe en estas Instrucciones de Uso.
Antes de cada utilización debe insertar una aguja nueva. Utilice únicamente las agujas compatibles con la pluma Semglee (ver “Instrucciones de Uso”).
Antes de cada inyección debe realizar una prueba de seguridad.
Inspeccione el cartucho antes de utilizar la pluma. No use Semglee si observa partículas en su interior. Solo utilice Semglee si la solución es transparente e incolora. No agitar ni mezclar antes de su uso.
Para prevenir la posible transmisión de enfermedades, nunca comparta su pluma con nadie más. Esta pluma es únicamente para su uso.
Utilice siempre una pluma nueva si nota que su control de azúcar en sangre empeora de manera inexplicable. Si usted piensa que podría tener un problema con la pluma Semglee, consulte con su médico, farmacéutico o enfermero.
Las plumas vacías no se deben rellenar y se deben desechar de forma segura.
No use la pluma Semglee si está dañada o no funciona correctamente (debido a defectos mecánicos), se debe desechar y utilizar una pluma Semglee nueva.
Confusiones de insulina
Debe comprobar siempre la etiqueta de insulina antes de cada inyección para evitar confusiones entre Semglee y otras insulinas.
Si usa más Semglee del que debe
Si se ha inyectado demasiado Semglee,su nivel de azúcar en sangre puede llegar a ser muy bajo (hipoglucemia). Compruebe su nivel de azúcar en sangre frecuentemente. En general, para prevenir la hipoglucemia debe comer más y controlar su nivel de azúcar en sangre. Para más información sobre el tratamiento de la hipoglucemia, ver el recuadro que aparece al final del prospecto.
Si olvidó usar Semglee
Si ha olvidado una dosis de Semglee o si no se ha inyectado suficiente insulina, su nivel de azúcar en sangre puede aumentar mucho (hiperglucemia). Compruebe su nivel de azúcar en sangre frecuentemente. Para más información sobre el tratamiento de la hiperglucemia, ver el recuadro que aparece al final del prospecto.
No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Semglee
Esto podría producir hiperglucemia grave (niveles muy altos de azúcar en sangre) y cetoacidosis (aumento del ácido en la sangre porque el organismo degrada las grasas en lugar del azúcar). No interrumpa su tratamiento con Semglee sin consultar con su médico, él le dirá lo que debe hacer.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Semglee puede producir efectos adversos, aunque no todas las personas los sufran.
Si nota signos de que su nivel de azúcar en sangre es demasiado bajo (hipoglucemia),actúe inmediatamente para subir su nivel de azúcar en sangre (ver el recuadro que aparece al final de este prospecto). La hipoglucemia (nivel bajo de azúcar en sangre) puede ser muy grave y es muy frecuente durante el tratamiento con insulina (puede afectar a más de 1 de cada 10 personas). Nivel bajo de azúcar en sangre significa que no hay suficiente azúcar en sangre. Si su nivel de azúcar en sangre baja mucho, se puede desmayar (perder el conocimiento). Una hipoglucemia grave puede provocar daños en el cerebro y puede ser potencialmente mortal. Para más información, ver el recuadro que aparece al final de este prospecto.
Reacciones alérgicas graves(raras, pueden afectar hasta 1 de cada 1.000 personas): los signos pueden incluir reacciones cutáneas a gran escala (erupción cutánea y picor por todo el cuerpo), hinchazón grave de la piel o de las membranas mucosas (angioedema), dificultad para respirar, descenso de la tensión arterial con latido cardiaco rápido y sudoración. Las reacciones alérgicas graves a las insulinas pueden ser potencialmente mortales. Informe a su médico inmediatamente si nota los signos de reacciones alérgicas graves.
Cambios de la piel en el lugar de inyección:
Si se inyecta insulina con demasiada frecuencia en el mismo lugar, la piel puede encogerse (lipoatrofia, puede afectar hasta 1 de cada 100 personas) o engrosarse (lipohipertrofia, puede afectar hasta 1 de cada 10 personas). También pueden aparecer bultos debajo de la piel causados por la acumulación de una proteína llamada amiloide (amiloidosis cutánea, cuya frecuencia es desconocida). La insulina podría no actuar muy bien. Cambie el lugar de inyección con cada inyección para ayudar a prevenir estos cambios de la piel.
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas)
- Efectos adversos de la piel y reacciones alérgicas en el punto de inyección
Los signos pueden incluir enrojecimiento, dolor intenso al inyectar poco habitual, picor, urticaria, hinchazón o inflamación. Estas reacciones pueden extenderse alrededor del punto de inyección. La mayor parte de las reacciones leves a la insulina desparecen habitualmente en unos días o en pocas semanas
- Efectos adversos de la piel y reacciones alérgicas en el punto de inyección
Los signos pueden incluir enrojecimiento, dolor intenso al inyectar poco habitual, picor, urticaria, hinchazón o inflamación. Estas reacciones pueden extenderse alrededor del punto de inyección. La mayor parte de las reacciones leves a la insulina desparecen habitualmente en unos días o en pocas semanas.
Efectos adversos raros(pueden afectar hasta 1 de cada 1.000 personas)
- Reacciones oculares
Un cambio significativo (mejoría o empeoramiento) del control de su nivel de azúcar en sangre puede alterar temporalmente su visión. Si padece una retinopatía proliferativa (una enfermedad de la vista relacionada con la diabetes) los ataques hipoglucémicos graves pueden provocar una pérdida temporal de la visión.
- Trastornos generales
En casos raros, el tratamiento con insulina puede provocar también una retención temporal de agua en el organismo, con hinchazón de las pantorrillas y los tobillos.
Efectos adversos muy raros(pueden afectar hasta 1 de cada 10.000 personas)
En casos muy raros, se puede producir disgeusia (trastornos del gusto) y mialgia (dolores musculares).
Uso en niños y adolescentes
En general, los efectos adversos en niños y adolescentes de 18 años o menores son similares a los aparecidos en adultos.
Se han comunicado con más frecuencia reclamaciones sobre reacciones en el lugar de inyección (reacción en el lugar de inyección, dolor en el lugar de inyección) y reacciones de la piel (erupción, urticaria) en niños o adolescentes de 18 años o menores que en adultos.
No existe experiencia en niños menores de 2 años.
Comunicación de efectos adversos
Si experimenta efectos adversos, consulte a su médico o farmacéutico. Esto incluye posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Semglee
Mantener este medicamento fuera de la vista y del alcance de los niños.
No use este medicamento después de la fecha de caducidad que aparece en el envase y en la etiqueta de la pluma después de CAD/EXP. La fecha de caducidad es el último día del mes que se indica.
Plumas sin utilizar
Conservar en nevera (entre 2 °C y 8 °C). No congelar ni colocar cerca del compartimento del congelador o de un acumulador de frío.
Mantener la pluma precargada en el embalaje exterior para protegerla de la luz.
Plumas en uso
La pluma precargada en uso o para llevarla como reserva, debe conservarse durante un máximo de 4 semanas por debajo de 30 °C y protegida del calor directo o de la luz directa. No utilizar después de este periodo de tiempo. La pluma en uso no debe guardarse en una nevera.
La tapa de la pluma se debe volver a colocar después de cada inyección para protegerla de la luz.
Retire la aguja después de la inyección y guarde la pluma sin la aguja. Asimismo, asegúrese de retirar la aguja antes de desechar la pluma. Las agujas no se deben volver a utilizar.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Semglee
- El principio activo es insulina glargina. Cada ml de solución contiene 100 unidades de insulina glargina (equivalente a 3,64 mg).
- Los demás componentes son: cloruro de zinc, metacresol, glicerol, hidróxido de sodio (para ajustar el pH) (ver sección 2 “Semglee contiene sodio”), ácido clorhídrico (para ajustar el pH) y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Semglee 100 unidades/ml solución inyectable en pluma precargada es una solución transparente incolora.
Cada pluma contiene 3 ml de solución inyectable (equivalente a 300 unidades).
Semglee está disponible en envases de 1, 3, 5 y 10 plumas o multienvase conteniendo 2 estuches, cada uno conteniendo 5 plumas.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Biosimilar Collaborations Ireland Limited
Unit 35/36
Grange Parade,
Baldoyle Industrial Estate,
Dublín 13
DUBLÍN
Irlanda
D13 R20R
Fabricante
Biosimilar Collaborations Ireland Limited
Block B, The Crescent Building, Santry Demesne
Dublín
D09 C6X8
Irlanda
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Biocon Biologics Belgium BV Tél/Tel: 0080008250910 | Lietuva Biosimilar Collaborations Ireland Limited Tel: 0080008250910 |
???????? Biosimilar Collaborations Ireland Limited ???: 0080008250910 | Luxembourg/Luxemburg Biocon Biologics France S.A.S Tél/Tel: 0080008250910 |
Ceská republika Biocon Biologics Germany GmbH Tel: 0080008250910 | Magyarország Biosimilar Collaborations Ireland Limited Tel.: 0080008250910 |
Danmark Biocon Biologics Finland OY Tlf: 0080008250910 | Malta Biosimilar Collaborations Ireland Limited Tel.: 0080008250910 |
Deutschland Biocon Biologics Germany GmbH Tel: 0080008250910 | Nederland Biocon Biologics France S.A.S Tel: 0080008250910 |
Eesti Biosimilar Collaborations Ireland Limited Tel: 0080008250910 | Norge Biocon Biologics Finland OY Tlf: +47 800 62 671 |
Ελλ?δα Biocon Biologics Greece ΜΟΝΟΠΡΟΣΩΠΗ Ι.Κ.Ε Τηλ.: 0080008250910 | Österreich Biocon Biologics Germany GmbH Tel: 0080008250910 |
España Biocon Biologics Spain S.L. Tel: 0080008250910 | Polska Biosimilar Collaborations Ireland Limited Tel: 0080008250910 |
France Biocon Biologics France S.A.S Tel: 0080008250910 | Portugal Biocon Biologics Spain S.L. Tel: 0080008250910 |
Hrvatska Biocon Biologics Germany GmbH Tel: 0080008250910 | România Biosimilar Collaborations Ireland Limited Tel: 0080008250910 |
Ireland Biosimilar Collaborations Ireland Limited Tel: 1800 777 794 | Slovenija Biosimilar Collaborations Ireland Limited Tel: 0080008250910 |
Ísland Biocon Biologics Finland OY Sími: +345 8004316 | Slovenská republika Biocon Biologics Germany GmbH Tel: 0080008250910 |
Italia Biocon Biologics Spain S.L. Tel: 0080008250910 | Suomi/Finland Biocon Biologics Finland OY Puh/Tel: 99980008250910 |
Κ?προς Biosimilar Collaborations Ireland Limited Τηλ: 0080008250910 | Sverige Biocon Biologics Finland OY Tel: 0080008250910 |
Latvija Biosimilar Collaborations Ireland Limited Tel: 0080008250910 |
Fecha de la última revisión de este prospecto: {mes AAAA}.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.

Semglee 100 unidades/ml solución inyectable en pluma precargada. INSTRUCCIONES DE USO
Lea cuidadosamente estas Instrucciones de Uso y el prospecto antes de usar la pluma precargada Semglee y cada vez que utilice otra pluma. Puede contener nueva información. Esta información no reemplaza la consulta con su médico, enfermero o farmacéutico acerca de su condición médica o tratamiento. Si no puede leer o seguir todas las instrucciones por su cuenta, pida ayuda a alguien que esté capacitado para usar esta pluma. No se recomienda que personas invidentes o con discapacidad visual utilicen esta pluma sin la ayuda de alguien capacitado para usarla.
Si no sigue estas instrucciones cada vez que usa la pluma, puede inyectarse demasiada insulina o demasiado poca. Esto puede afectar su nivel de azúcar en sangre.
Semglee es un inyector de pluma precargada desechable que contiene 300 unidades de insulina glargina en 3 ml de solución (100 unidades/ml). Puede inyectarse de 1 a 80 unidades en una sola inyección.
No comparta la pluma precargada Semglee con otra persona, incluso aunque se haya cambiado la aguja. Puede transmitir a otros una infección grave o contagiarse a través de ellos.
Montaje de la pluma:
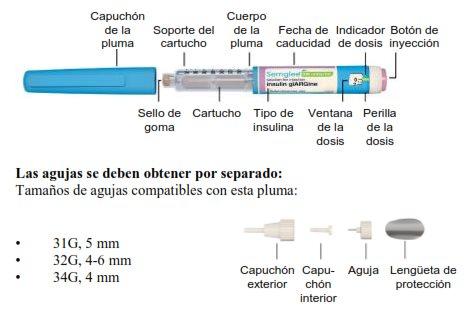
Suministros requeridos:
Asegúrese de tener los siguientes elementos antes de inyectarse su dosis:
- Pluma Semglee
- Aguja hipodérmica desechable estéril compatible con esta pluma
- 2 paños con alcohol
- Recipiente para eliminación de objetos punzantes
Almacenamiento
Antes de usar la pluma por primera vez, guarde los envases que contienen la pluma en la nevera
(2 °C–8 °C).
Nocongele la pluma.
Después de sacar una pluma de la nevera, apóyela sobre una superficie plana y espere a que alcance la temperatura ambiente, entre 15 °C y 30 °C, antes de usarla.
Después del primer uso de la pluma, consérvela a temperatura ambiente hasta un máximo de
30 °C. No vuelva a colocar la pluma en la nevera después de usarla.
Guarde siempre la pluma con el capuchón puesto para evitar la contaminación.
Debe desechar la pluma que está utilizando después de 4 semanas del primer uso, incluso si todavía le queda insulina. Consulte el Paso 8 para ver las instrucciones de eliminación.
Nodeje la aguja colocada en la pluma durante el almacenamiento ni reutilice las agujas. Mantenga la pluma y las agujas fuera de la vista y del alcance de los niños.
Utilice siempre una aguja nueva y estéril para cada inyección, ya que esto impide que haya agujas bloqueadas y evita las infecciones.
Cada vez que use la pluma
- Lávese las manos con agua y jabón antes de usar la pluma.
- Revise la etiqueta de la pluma para asegurarse de que se va a inyectar el tipo correcto de insulina. La pluma tiene una etiqueta violeta y blanca y un botón de inyección violeta.
- Revise la fecha de caducidad en la etiqueta de la pluma. No la use una vez transcurrida esta
fecha.
- Compruebe que el medicamento en el cartucho de la pluma se vea transparente e incoloro. No use la pluma si el medicamento en el interior del cartucho está opaco, con color o si puede ver partículas.
- Utilice siempre una aguja nueva desechable y estéril para cada inyección.
- Utilice un punto de inyección que le haya indicado el profesional sanitario que le atiende.
Paso 1. Preparación de la pluma
A – Inspeccione la pluma: revise la etiqueta violeta y blanca de la pluma para asegurarse de que:
- Es el tipo de insulina correcto.
- No se ha superado la fecha de caducidad.
B – Sostenga el cuerpo de la pluma con una mano. Retire el capuchón de la pluma con la otra mano. Deje el capuchón de lado para usarlo posteriormente.
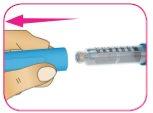
C – Revise la insulina a través del soporte del cartucho para asegurarse de que:
- El aspecto de la insulina es transparente e incoloro.
- No se observan grietas, roturas ni fugas alrededor del soporte del cartucho.
D – Limpie el sello de goma (en la parte delantera del cartucho) con un paño con alcohol nuevo.
Paso 2. Colocación de una aguja nueva
A – Tome una nueva aguja desechable y estéril y desprenda el sello protector. Noutilice la aguja si el sello protector está dañado o falta, ya que la aguja puede estar contaminada.
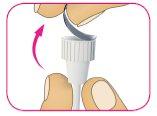
B – Mientras sostiene el cuerpo de la aguja mirando hacia arriba, coloque el capuchón exterior de la aguja directamente sobre el soporte del cartucho, tal como se muestra. Si intenta colocar el capuchón exterior lateralmente, la aguja puede doblarse o dañarse.
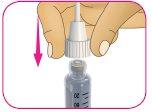
C – Gire el capuchón exterior de la aguja en el sentido de las agujas del reloj (hacia la derecha) hasta que sienta que se ajusta firmemente en la pluma.
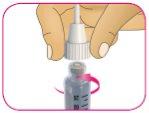
D – Retire con cuidado el capuchón exterior de la aguja y déjelo de lado. No lo tire, ya que lo necesitará después.
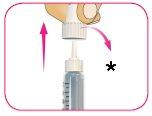
*Conserve el capuchón exterior
E – Retire con cuidado el capuchón interior de la aguja y deséchelo.
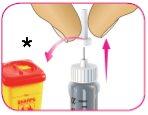
- Tire el capuchón interior
Paso 3. Preparación de la aguja de la pluma
A – Prepare siempre toda nueva aguja de la pluma antes de cada inyección.
B – Gire la perilla de la dosis blanca a 2 unidades de dosis. Escuchará un “clic” por cada unidad que
gira.
Si gira más allá de 2 unidades por error, vuelva a girar la perilla de la dosis en dirección opuesta para corregir el número de unidades.
B – Sostenga el cuerpo de la pluma mirando hacia arriba con una mano.
D – Golpee suavemente el cartucho con el dedo para ayudar a que las burbujas de aire grandes se desplacen hacia la parte superior del cartucho. Es posible que todavía se vean burbujas pequeñas. Esto es normal.
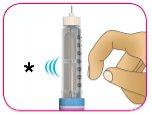
- GOLPEE
E – Con la pluma recta, presione el botón de inyección hasta que deje de moverse y la ventana de la
dosis muestre “0”.
F – Repita los pasos del 3B al 3E hasta tres veces más hasta que vea gotas de insulina en la punta de la aguja.
La preparación finaliza cuando puede ver las gotas de insulina.

Si no ve insulina en la punta de la aguja después de 4 intentos de preparación, la aguja puede estar tapada. Si esto ocurre:
- Vaya al paso 7 para ver instrucciones sobre cómo retirar la aguja de forma segura.
- Vuelva a empezar el proceso en el paso 2A para colocar y preparar una aguja nueva.
Paso 4. Selección de la dosis
A – Compruebe que en la ventana de la dosis aparece “0”.
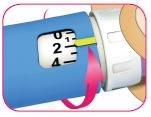
B – Gire la perilla de la dosis blanca hasta que el indicador de dosis amarillo quede alineado con la dosis deseada.
A medida que gira la perilla de la dosis blanca para ajustar su dosis, esta se extenderá y escuchará un
“clic” en cada unidad marcada.
La dosis se puede corregir; para ello, debe girar la perilla de la dosis en cualquier dirección hasta que la dosis correcta quede alineada con el indicador de dosis amarillo.
 Ejemplo de 48 unidades seleccionadas
Ejemplo de 48 unidades seleccionadas
La pluma no le permitirá marcar una dosis que supere el número de unidades que quedan en la pluma. Si su dosis supera el número de unidades que quedan en la pluma:
- Inyéctese la cantidad que queda en la pluma y use una pluma nueva para administrarse el resto de la dosis
o
- Tome una pluma nueva e inyéctese la dosis completa.
Nofuerce la perilla de la dosis más allá de 80 unidades.
Nopresione el botón de inyección violeta mientras gira la perilla de la dosis.
Paso 5. Selección y limpieza del punto de inyección
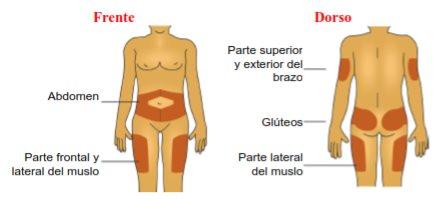
A – Seleccione el punto de inyección tal como se lo haya explicado el profesional sanitario, limpie la zona con un paño con alcohol nuevo y deje secar la piel antes de inyectarse la dosis.
Los puntos de inyección incluyen los brazos, los muslos, los glúteos y el abdomen. Debe cambiar los puntos de inyección para cada inyección.
Paso 6. Inyección de la dosis
A – Si se lo ha indicado el profesional sanitario, puede pellizcar la piel limpia entre sus dedos.
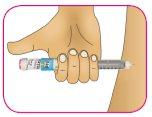
B – Introduzca la aguja en la piel de forma recta, tal como se lo ha indicado el profesional sanitario.
Nose inyecte con la aguja en ángulo.
C – Presione el botón de inyección violeta hasta el fondo. La perilla de la dosis blanca girará y
escuchará unos “clics” a medida que presiona.
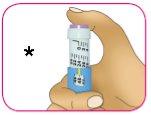
- Presione para administrar
D – Mantenga presionado el botón de inyección violeta durante 10 segundos después de que aparezca “0” en la ventana de la dosis para asegurarse de que se inyecta toda la insulina. Si no mantiene presionado el botón de inyección durante 10 segundos después de que aparezca “0”, puede recibir la dosis incorrecta del medicamento.
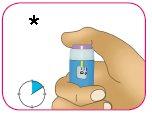
- Mantenga presionado durante 10 segundos
Nopresione el botón de inyección lateralmente ni bloquee la perilla blanca de la dosis con sus dedos, ya que esto le impedirá inyectarse el medicamento.
Paso 7. Después de la inyección
A – Tome el capuchón exterior de la aguja que apartó en el paso 2D, sosténgalo por la parte más ancha y cubra la aguja con cuidado, sin tocarla.

B – Apriete la parte ancha del capuchón exterior de la aguja y desenrosque la aguja en sentido contrario a las agujas del reloj (hacia la izquierda). Siga girando la aguja hasta que salga de la pluma. Es posible que deba dar varias vueltas para liberar la aguja.
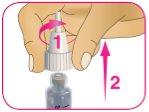
C – Coloque la aguja en el recipiente para eliminación de objetos punzantes (consulte el Paso 8 para ver las instrucciones de eliminación).

D – Vuelva a colocar el capuchón de la pluma sobre el cartucho.
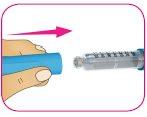
E – Guarde la pluma a temperatura ambiente (por debajo de 30 °C). Noguarde la pluma con una aguja usada colocada.
Paso 8. Eliminación
Coloque la aguja usada en un recipiente para eliminación de objetos punzantes inmediatamente después del uso. Notire (deseche) agujas sueltas en un recipiente de residuos doméstico.
Si no posee un recipiente para objetos punzantes, puede usar un recipiente doméstico que:
- esté fabricado en plástico resistente,
- pueda cerrarse con una tapa hermética resistente a las perforaciones, que no permita la salida de los objetos punzantes,
- se mantenga recto y estable durante el uso,
- sea a prueba de fugas y
- esté debidamente etiquetado para advertir sobre desechos peligrosos en el interior del recipiente.
La pluma usada se puede desechar en el recipiente de residuos domésticos una vez que ha retirado la aguja.
Cuidado de la pluma
- Lleve siempre un inyector de pluma precargada de insulina adicional según lo recomendado por el profesional sanitario que le atiende, en caso de que su pluma se pierda o sufra daños.
- Utilice siempre una aguja nueva desechable y estéril para cada inyección.
- Mantenga la pluma alejada de la humedad, el polvo, la luz directa del sol y lugares donde la temperatura pueda subir o bajar demasiado (consulte la sección de almacenamiento al principio de estas instrucciones).
- Puede limpiar la parte exterior de su pluma con un trapo húmedo.
- Evite que la pluma se caiga, ya que esto puede hacer que el cartucho se rompa o puede dañar la pluma.
- Nocomparta su pluma con otras personas, incluso aunque se haya cambiado la aguja. Puedetrasmitir a otros una infección grave o contagiarse de ellos.
- Noponga en remojo ni lave la pluma. Nouse alcohol, peróxido de hidrógeno, lejía ni ningún otro líquido para limpiar la pluma. Noaplique lubricantes, como aceite. Esto podría dañar la pluma.
- Nointente arreglar una pluma inutilizable o dañada. Retire la aguja según lo descrito en el paso 7 y deseche la pluma o devuélvala a su farmacéutico. Use una pluma nueva en su lugar.
- País de registro
- Precio medio en farmacia56.25 EUR
- Disponibilidad en farmacias
Problema de suministro reportado
Los datos de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) indican un problema de suministro que afecta a este medicamento.<br><br>La disponibilidad puede ser limitada en algunas farmacias.<br><br>Para actualizaciones o alternativas, consulte a su farmacéutico. - Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a SEMGLEE 100 UNIDADES/ML SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 100 U/mlPrincipio activo: insulina glarginaFabricante: Eli Lilly Nederland B.V.Requiere recetaForma farmacéutica: INYECTABLE, DesconocidaPrincipio activo: insulina glarginaFabricante: Sanofi-Aventis Deutschland GmbhRequiere recetaForma farmacéutica: INYECTABLE, DesconocidaPrincipio activo: insulina glarginaFabricante: Sanofi-Aventis Deutschland GmbhRequiere receta
Médicos online para SEMGLEE 100 UNIDADES/ML SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de SEMGLEE 100 UNIDADES/ML SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes

















