
RUCONEST 2100 U POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use RUCONEST 2100 U POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Ruconest 2100 units powder and solvent for solution for injection
conestat alfa
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Ruconest and what is it used for
- What you need to know before you use Ruconest
- How to use Ruconest
- Possible side effects
- Storage of Ruconest
- Contents of the pack and other information
1. What is Ruconest and what is it used for
Ruconest contains conestat alfa as the active substance. Conestat alfa is a recombinant (non-blood-derived) form of human C1 inhibitor (rhC1-INH).
Ruconest should be used by adults, adolescents, and children (from 2 years of age) with a rare hereditary blood disorder called hereditary angioedema (HAE). These patients have a deficiency of the C1 inhibitor protein in their blood, which can cause repeated episodes of swelling, abdominal pain, difficulty breathing, and other symptoms.
Administration of Ruconest resolves the C1 inhibitor deficiency and allows a reduction of the symptoms of acute HAE attacks.
2. What you need to know before you use Ruconest
Do not use Ruconest:
- If you are or are considered allergic to rabbits
- If you are allergic to conestat alfa or any of the other components of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor before starting to use Ruconest.
If you experience allergic reactions, such as hives, rash, itching, dizziness, wheezing, difficulty breathing, or swelling of the tongue after administration of Ruconest, seek emergency medical assistance to urgently treat the symptoms of the allergic reaction.
Children and adolescents
Do not administer this medicine to children under 2 years of age. Ruconest has not been studied in children under 5 years of age. Your doctor will determine if treatment with Ruconest is suitable for your child. Additional monitoring of your child is required to detect symptoms of allergic reactions during and after administration.
Using Ruconest with other medicines
Tell your doctor if you are using, have recently used, or might use any other medicines.
If you receive tissue plasminogen activator as acute treatment for the prevention of blood clots (anticoagulant treatment), you should not use Ruconest at the same time.
Pregnancy and breastfeeding
Ruconest should not be administered during pregnancy or breastfeeding.
If you plan to become pregnant, consult your doctor before using Ruconest.
Driving and using machines
Do not drive or operate machines if you feel dizzy or have a headache after using Ruconest.
Ruconest contains sodium (19.5 mg per vial)
Patients on low-sodium diets should be aware that this medicine contains 19.5 mg of sodium per vial.
3. How to use Ruconest
Treatment with Ruconest will be initiated by a doctor specialized in the diagnosis and treatment of hereditary angioedema.
Ruconest should be administered by a healthcare professional until you or your caregiver have received the necessary training and are able to administer Ruconest.
Always use this medicine exactly as described in this leaflet or as directed by your doctor or nurse. Consult your doctor or nurse if you are unsure.
Ruconest is administered into a vein over approximately 5 minutes. Your dose will be based on your body weight.
Most of the time, one dose is sufficient. An additional dose may be administered if your symptoms do not improve after 120 minutes (for adults and adolescents) or 60 minutes (for children). No more than two doses, calculated according to step 7, should be administered within a 24-hour period.
You or your caregiver can inject Ruconest only after receiving adequate instructions and necessary training from your doctor or nurse.
Instructions for use
Do not mix or administer Ruconest with other medicines or solutions. The following describes how to prepare and administer the Ruconest solution.
Before you start
- Make sure the packaging is intact and contains all the components specified in section 6 of this leaflet.
- In addition to the packaging, you will need:
- A tourniquet
- Adhesive tape to secure the needle
- Inspect the vials and other components.
- All vials must be sealed with a plastic cap and an aluminum stopper without visible damage, such as cracks in the glass.
- Check the expiration date. Do not use any component of the kit after the expiration date indicated on the large outer packaging.
In a single box, the different components may have different expiration dates. The expiration date of the outer packaging reflects the expiration date of the component with the shorter validity period.
- Wait until the required number of powder and solvent vials reach room temperature.
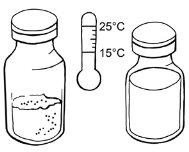
Preparation of the solution
Step 1:Cleaning and other requirements
- Wash your hands thoroughly.
- Place the required powder and solvent vials on a flat and clean surface.
- Body weight of 42 kg or less: 1 vial of powder and 1 vial of solvent
- Body weight over 42 kg: 2 vials of powder and 2 vials of solvent
- Place the vial adapters on the work surface. Do not remove the packaging from the adapters.
- 2 adapters for 1 vial of powder and 1 vial of solvent
- 4 adapters for 2 vials of powder and 2 vials of solvent
- Place the syringes on the work surface. Do not remove the packaging from the syringes.
- 1 syringe for 1 vial of powder and 1 vial of solvent
- 2 syringes for 2 vials of powder and 2 vials of solvent
Step 2:Disinfection of vial stoppers
- Remove the plastic pressure cap from the powder and solvent vials.
- Use an alcohol swab to disinfect all vial stoppers and wait at least 30 seconds until the stoppers are dry.
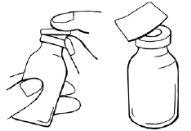
- After disinfection, do not touch the stoppers with your hands or any object.
Step 3:Assembly of adapters on vials
- Hold a packaged adapter with one hand and remove the cap. The adapter should remain in its plastic packaging.
- Place the adapter on a powder vial and pierce the stopper until it is secured to the neck of the vial.
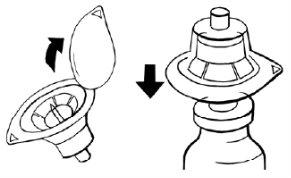
- Leave the adapter packaging on until you connect the syringe according to steps 4 and 5.
- Repeat the above steps to assemble an adapter on the solvent vial. All adapters supplied in the packaging are identical.
- If you need to use a second powder and solvent vial, repeat the above steps.
Step 4:Withdrawal of solvent
- Remove a sterile syringe from its packaging.
- Remove the adapter packaging from the solvent vial.
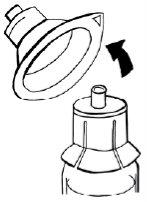
- Hold the adapter with one hand. With the other hand, connect the syringe and turn it to the left until it stops to secure it.
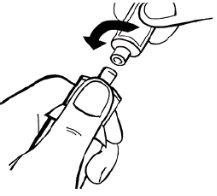
- Invert the solvent vial completely along with the adapter and syringe. While keeping it in a vertical position, slowly inject 14 ml of solvent.
If bubbles form, minimize them as much as possible. To do this, give a light tap on the syringe and apply gentle pressure by pushing the plunger in the syringe. Continue filling the syringe until you reach the 14 ml of solvent.
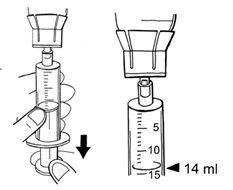
- To disconnect the syringe from the adapter, turn it to the left.
- Leave the remaining solvent in the vial and discard the vial.
- Place the syringe on the work surface and avoid touching the surface or any other object with the tip of the syringe.
Step 5:Addition of solvent to powder and dissolution
- Remove the adapter packaging from the powder vial.
- Take the syringe with solvent that you prepared in step 4.
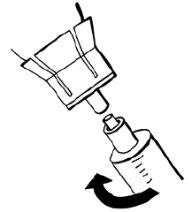
- Hold the adapter with the other hand and connect the syringe. To secure the syringe, turn it to the right until it stops.
- Slowly press the solvent with a single movement to introduce it into the powder vial and minimize foam formation.
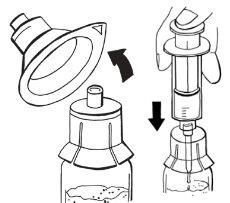
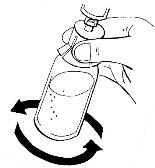
- Leave the syringe in the adapter and carefully turn the vial for about half a minute. Do not shake it.
After turning, leave the vial on the surface for several minutes until the solution is clear. If there is undissolved powder, repeat the procedure.
- Repeat steps 4 and 5 if you need to prepare a second solution.
Step 6:Checking prepared solutions
- Check if the powder from the vials has dissolved completely and if the plunger is at the bottom of the syringe.
- Once the powder is dissolved, the solution should be clear and colorless.
- Do not use the prepared solution if it is cloudy, contains particles, or has changed color. Inform the healthcare professional in this case. A small amount of foam formation is acceptable.
Step 7:Withdrawal of prepared solution
- Calculate the milliliters of prepared solution to be injected.
Body weight | Milliliters of prepared solution to be injected |
Less than 84 kg | Body weight in kg divided by three |
84 kg and over | 28 ml |
- Inject the volume of prepared solution while keeping the syringe in a vertical position. If you have prepared:
- one vial with solution, withdraw the calculated volume
- two vials and your body weight is less than 84 kg, withdraw a similar amount:
- 14 ml from the first vial
- from the second vial, the difference between the calculated volume and the 14 ml from the first vial
- two vials and your body weight is 84 kg or more, withdraw 14 ml from each vial in each syringe
If bubbles form, minimize them as much as possible. To do this, give a light tap on the syringe and apply gentle pressure by pushing the plunger in the syringe. Continue filling the syringe until you reach the required volume.
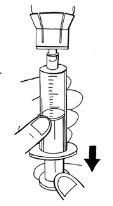
- Never exceed the volume of 14 ml per syringe.
- To release the syringe, turn it to the left and discard the vials along with the adapter.
- Place the syringe on the work surface and avoid touching the surface or any other object with the tip of the syringe.
Step 8:Checking prepared syringes
- Re-check if the volume of the syringes prepared in step 7 is correct.
Administration into a vein
It is very important that the prepared solution is injected directly into a vein and not into an artery or the surrounding tissue.
Inject the Ruconest solution immediately after preparation, preferably while seated.
Step 9:Required components
- Check if all necessary components are on the work surface:
- 1 or 2 syringes with the prepared solution
- 1 infusion set with a 25 G needle
- 1 alcohol swab
- 1 non-woven sterile gauze
- 1 self-adhesive dressing
- 1 tourniquet
- 1 adhesive tape to secure the needle
Step 10:Preparation of the infusion set
- Remove the screw cap from the end of the infusion set. This is the end without the needle.
- Hold this end with one hand, connect the syringe tip, and secure it by turning it to the right until it stops.
- Hold the syringe with the tip upwards. Gently press the syringe plunger to carefully fill the infusion set with the prepared solution.
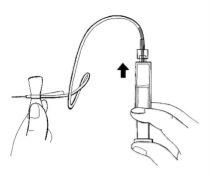
- Check that there is no air in the syringe, infusion tube, or needle.
Step 11:Preparation of the injection site
- Place the tourniquet over the injection site, preferably in the middle of the upper arm. Tighten to compress the vein. You can achieve this effect by clenching your fist.
- Palpate the suitable vein with the other hand.
- Disinfect the injection site well with an alcohol swab and let the skin dry.
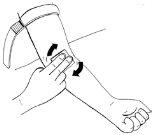
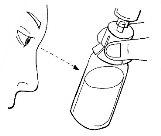
Step 12:Administration of the prepared solution
- Remove the needle cover.
- Insert the infusion set needle carefully, at the most shallow angle possible, into the vein.
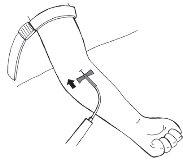
- Secure the needle with the adhesive tape, approximately 7 cm long, over the wings of the needle.
- Gently and slightly pull back the syringe plunger until you see blood enter the tube to ensure the needle is in the vein.
- Release the tourniquet.
- If there is no blood in the tube, remove the needle, repeat all steps from step 11, and reinsert the needle.
- If there is blood, carefully inject the solution into the vein, as shown in the image. Inject over approximately 5 minutes.
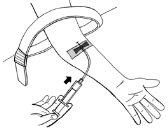
- If you have prepared two syringes:
- Fold the tube near the connector of the infusion set to avoid flow inversion
- Unscrew the empty syringe from the infusion set and immediately replace it with the second syringe.
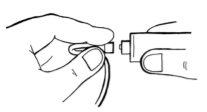
- Unfold the tube and carefully inject this solution in a similar way to the first syringe.
Step 13:After administration
- Carefully remove the adhesive tape that secures the needle and remove the needle from the vein.
- Immediately after removing the needle, press the sterile gauze over the injection site for a few minutes to reduce bleeding.
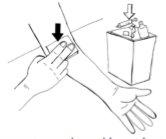
- Then, place the self-adhesive dressing over the injection site.
- Fold the yellow protective cap over the needle.
- Safely discard the used infusion set with the needle, unused solution, syringe, and empty vial in an appropriate waste container, as these materials can cause injury if not disposed of properly. Do not reuse the equipment.
Step 14:Documentation of administration
Record the following (for example, in your diary):
- Date and time of administration
- Batch number printed on the label of the powder vial
If you use more Ruconest than you should
Contact your doctor or the nearest hospital.
If you have any further questions on the use of this medicine, ask your doctor.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
If your symptoms worsen and/or you present with a rash, tingling, difficulty breathing, or swelling of the face or tongue, consult your doctor immediately. These symptoms may indicate that you have developed an allergy to Ruconest.
During treatment with Ruconest, some side effects may appear:
Frequent: may affect up to 1 in 10 people
- Nausea
Infrequent: may affect up to 1 in 100 people
- Abdominal pain, diarrhea
- Sensation of tingling, pinching, or numbness of the mouth
- Headache, dizziness
- Decreased sense of touch or sensitivity in the skin or extremities
- Throat irritation
- Urticaria (hives)
- Swelling of the ears or the area around the ears
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, even if it is a possible adverse effect that does not appear in this prospectus. You can also report it directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Ruconest
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the packaging and on the label of the vial after EXP. The expiration date is the last day of the month indicated.
Do not store at a temperature above 25°C.
Keep the powder vial in the vial box to protect it from light.
Before administering Ruconest, it must be dissolved in the solvent included in the packaging (see section 3). Once reconstituted, the product must be used immediately.
Do not use this medicine if, after dissolution, you observe that the solution contains particles or if the solution is discolored. The formation of foam is acceptable in small quantities.
6. Package Contents and Additional Information
Composition of Ruconest
Powder vial:
- The active ingredient is conestat alfa. One powder vial contains 2100 units (U) of conestat alfa. This is equivalent to 2100 units per 14 ml after reconstitution, or a concentration of 150 units/ml.
- The other components are sucrose, sodium citrate (E331), and citric acid.
Solvent vial:
- The solvent ingredient is water for injectable preparations.
Appearance of the Product and Package Contents
Ruconest is presented in a single glass vial containing a white to off-white powder for injectable solution, along with a glass vial with a clear, colorless solvent to dissolve the powder. After dissolving the powder in water for injectable preparations, the solution is clear and colorless.
Ruconest is supplied as a kit in a box that contains:
- 1 vial of 2100 U of powder
- 1 vial of 20 ml of solvent
- 2 vial adapters
- 1 syringe
- 1 infusion set with a 35 cm tube and a 25 G needle
- 2 alcohol swabs
- 1 non-woven sterile gauze
- 1 self-adhesive bandage
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Pharming Group N.V.
Darwinweg 24
2333 CR Leiden
Netherlands
Manufacturer:
Pharming Technologies B.V.
Darwinweg 24
2333 CR Leiden
Netherlands
Date of the Last Revision of this Prospectus:
Detailed information about this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu/.
------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
DOSAGE AND ADMINISTRATION
Dosage
Body weight up to 84 kg
- An intravenous injection of 50 U/kg of body weight.
Body weight of 84 kg or more
- An intravenous injection of 4200 U (two vials).
In most cases, a single injection of Ruconest is sufficient for the treatment of an acute angioedema crisis.
If the clinical response is insufficient, a second dose (50 U/kg of body weight up to 4200 U) may be administered.
No more than two doses can be administered within a 24-hour period.
Dose Calculation
Determine the patient's body weight.
Body weight up to 84 kg
- In patients weighing up to 84 kg, the administration volume required will be calculated using the following formula:
Volume to be Administered (ml) | = | Body Weight (kg) x 50 (U/kg) 150 (U/ml) | = | Body Weight (kg) 3 |
Body weight of 84 kg or more
- In patients weighing 84 kg or more, the administration volume required is 28 ml, equivalent to 4200 U (2 vials).
Reconstitute each vialwith 14 ml of water for injectable preparations (see Reconstitution section below).
The reconstituted solution in each vial contains 2100 U of conestat alfa at 150 U/ml.
The required volume of the reconstituted solution should be administered by slow intravenous injection over approximately 5 minutes.
SPECIAL PRECAUTIONS FOR DISPOSAL AND OTHER HANDLING
Preparation and Handling
Each vial of Ruconest is for single use.
Ruconest should be administered intravenously after reconstitution with water for injectable preparations. Aseptic technique should be used for reconstitution, combination, and mixing of solutions.
Reconstitution
- Each vial of Ruconest (2100 U) should be reconstituted with 14 ml of water for injectable preparations.
- Disinfect the rubber stoppers of the powder and solvent vials, and place a vial adapter on each vial of solvent and powder to connect it to the vial neck.
- Connect the syringe to the adapter of the solvent vial and turn it to the right until it is connected. To release the syringe from the adapter, turn it to the left and discard the vial along with the adapter.
- Connect the syringe with solvent to the adapter of the powder vial and turn it to the right until it is connected. The solvent should be added slowly to avoid strong impact on the powder and mixed gently to minimize foam formation in the solution. Leave the syringe in the adapter. Repeat steps 3 and 4 if you need to prepare a second solution (this requires a second package).
- The reconstituted solution contains 150 U/ml of conestat alfa and is a clear, colorless solution. The reconstituted solution in each vial should be examined for particles and color changes. Do not use a solution that presents particles or color changes. The formation of small amounts of foam is acceptable. This medicine should be used immediately.
Administration
- Inject the required volume of the prepared solution. Do not exceed 14 ml per syringe. To release the syringe, turn it to the right and discard the vial along with the adapter.
- Connect the infusion set to the syringe and turn it to the right to lock it. Hold the syringe with the tip upwards and gently press the plunger to fill the infusion set with solution.
- Disinfect the injection site with an alcohol swab. Remove the needle cover from the infusion set and carefully insert the needle into the vein.
- Make sure to release the tourniquet. Inject the solution into the vein carefully (over approximately 5 minutes).
- If preparing two syringes, fold the tube to avoid flow inversion, unscrew the empty syringe from the infusion set (to the left) and immediately replace it with the second syringe. Inject the solution from the second syringe carefully.
Disposal
Safely dispose of the used infusion set with the needle, unused solution, syringe, and empty vial in an appropriate medical waste container, as these materials can cause injury if not disposed of correctly. Do not reuse the equipment.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RUCONEST 2100 U POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 2100 UActive substance: conestat alfaManufacturer: Pharming Group N.V.Prescription requiredDosage form: INJECTABLE, 200 mgActive substance: Drugs used in hereditary angioedemaManufacturer: Csl Behring GmbhPrescription requiredDosage form: INJECTABLE, 1500 IUActive substance: c1-inhibitor, plasma derivedManufacturer: Csl Behring GmbhPrescription required
Online doctors for RUCONEST 2100 U POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about RUCONEST 2100 U POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















