REVESTIVE 5 MG POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar REVESTIVE 5 MG POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Revestive 5 mg polvo y disolvente para solución inyectable
teduglutida
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Revestive y para qué se utiliza
- Qué necesita saber antes de empezar a usar Revestive
- Cómo usar Revestive
- Posibles efectos adversos
- Conservación de Revestive
- Contenido del envase e información adicional
1. Qué es Revestive y para qué se utiliza
Revestive contiene el principio activo teduglutida. Mejora la absorción intestinal de nutrientes y líquidos de su tracto gastrointestinal (intestino).
Revestive se utiliza para el tratamiento del síndrome de intestino corto en adultos, niños y adolescentes (de 4 meses y mayores). El síndrome de intestino corto es un trastorno que se produce por una incapacidad de absorber los nutrientes de los alimentos y el agua en su recorrido por el intestino. Generalmente está causado por la extirpación quirúrgica parcial o total del intestino delgado.
2. Qué necesita saber antes de empezar a usar Revestive
No use Revestive
- si es alérgico a la teduglutida o a alguno de los demás componentes de este medicamento (incluidos en la sección 6) o a trazas de residuos de tetraciclinas;
- si padece o cree que puede padecer cáncer;
- si ha padecido cáncer en alguna parte del tracto gastrointestinal, como el hígado, la vesícula biliar o los conductos biliares y el páncreas, en los últimos cinco años.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Revestive:
- si padece insuficiencia hepática severa. Su médico lo tendrá en cuenta a la hora de recetarle este medicamento;
- si padece alguna enfermedad cardiovascular (que afecta al corazón y/o a los vasos sanguíneos), tales como presión sanguínea alta (hipertensión) o corazón débil (insuficiencia cardiaca). Los signos y síntomas incluyen aumento brusco de peso, hinchazón facial, hinchazón en los tobillos y/o dificultad para respirar;
- si presenta otras enfermedades severas que no están bien controladas. Su médico lo tendrá en cuenta a la hora de recetarle este medicamento;
- si padece insuficiencia renal. Es posible que su médico tenga que darle una dosis más baja de este medicamento.
En el momento de iniciar o mientras esté recibiendo el tratamiento con Revestive, su médico puede ajustar la cantidad de líquidos o nutrición por vía intravenosa que recibe.
Revisiones médicas antes y durante el tratamiento con Revestive
Antes de comenzar el tratamiento con este medicamento, su médico necesitará hacerle una colonoscopia (un procedimiento para observar el interior del colon y el recto) para detectar la presencia de pólipos (pequeños crecimientos anómalos) y eliminarlos. Se recomienda que su médico realice estas exploraciones una vez al año durante los 2 primeros años tras el inicio del tratamiento y después, como mínimo, una vez cada cinco años. Si se hallan pólipos antes o durante el tratamiento con Revestive, su médico decidirá si debe continuar usando este medicamento. Si durante la colonoscopia se le diagnostica cáncer, no debe usar Revestive. El médico vigilará sus electrolitos y líquidos corporales ya que un desequilibrio podría provocar sobrecarga de líquidos o deshidratación.
Su médico tendrá especial cuidado y hará un seguimiento de la función de su intestino delgado y de los signos y síntomas que indiquen problemas en su vesícula biliar, conductos biliares y páncreas.
Niños y adolescentes
Revisiones médicas antes y durante el tratamiento con Revestive
Antes de comenzar el tratamiento con este medicamento, se le realizará un análisis para ver si hay sangre en las heces. También se le realizará una colonoscopia (un procedimiento para observar el interior del colon y el recto para detectar la presencia de pólipos [pequeños crecimientos anómalos] y eliminarlos) si presenta sangre en las heces de causa no identificada. Si se hallan pólipos antes del tratamiento con Revestive, su médico decidirá si debe utilizar este medicamento. Si durante la colonoscopia se le diagnostica cáncer, no debe usar Revestive. Su médico le realizará más colonoscopias mientras siga recibiendo tratamiento con Revestive. El médico vigilará sus electrolitos y líquidos corporales ya que un desequilibrio podría provocar sobrecarga de líquidos o deshidratación.
Niños menores de 4 meses de edad
Este medicamento no debe utilizarse en niños menores de 4 meses de edad. No se dispone de datos con Revestive para este grupo de edad.
Otros medicamentos y Revestive
Informe a su médico, farmacéutico o enfermero si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Revestive puede afectar a la forma en que otros medicamentos se absorben en el intestino y, por tanto, reducir su eficacia. Es posible que su médico tenga que cambiarle la dosis de otros medicamentos.
Embarazo y lactancia
No se recomienda el uso de Revestive si está embarazada o en periodo de lactancia.
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico, farmacéutico o enfermero antes de utilizar este medicamento.
Conducción y uso de máquinas
Este medicamento puede provocar mareo. Si esto sucediera, no conduzca ni utilice máquinas hasta que se encuentre mejor.
Información importante sobre algunos componentes de Revestive
Este medicamento contiene menos de 23 mg de sodio (1 mmol) por dosis; esto es, esencialmente “exento de sodio”.
Hay que tener precaución si usted es hipersensible a las tetraciclinas (ver la sección “No use Revestive”).
3. Cómo usar Revestive
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico, farmacéutico o enfermero.
Dosis
La dosis diaria recomendada es 0,05 mg por kg de peso corporal. La dosis se dará en mililitros (ml) de solución.
Su médico decidirá la dosis más adecuada para usted dependiendo de su peso corporal. Su médico le indicará la dosis a inyectar. En caso de duda, consulte de nuevo a su médico, farmacéutico o enfermero.
Uso en niños y adolescentes
Revestive puede utilizarse en niños y adolescentes (de 4 meses o mayores). Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico.
Cómo usar Revestive
Revestive se inyecta bajo la piel (vía subcutánea) una vez al día. La inyección puede administrársela el propio paciente u otra persona, por ejemplo su médico, cuidador o enfermero domiciliario. Si se está inyectando el medicamento usted mismo, o se lo está inyectando su cuidador, tanto usted como su cuidador deben recibir la formación adecuada por parte de su médico o enfermero. Encontrará las instrucciones detalladas para la inyección al final de este prospecto.
Se recomienda encarecidamente que cada vez que usted o su hijo reciban una dosis de Revestive, se registren el nombre y el número de lote del medicamento a fin de mantener un registro de los lotes utilizados.
Si usa más Revestive del que debe
Si, de forma accidental, se inyecta más Revestive del indicado por su médico, debe ponerse en contacto con su médico, farmacéutico o enfermero.
Si olvidó usar Revestive
Si olvida usar este medicamento (o no puede usarlo a su hora habitual), utilícelo lo antes posible ese mismo día. Nunca utilice más de una inyección en el mismo día. No utilice una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Revestive
Continúe usando este medicamento durante el tiempo que su médico le indique. No deje de usar este medicamento sin consultarlo con su médico, ya que una interrupción repentina puede provocarle cambios en el equilibrio de líquidos.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Solicite atención médica inmediatamente si se produce alguno de los siguientes efectos adversos:
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- Insuficiencia cardiaca congestiva. Contacte con su médico si presenta cansancio, dificultad para respirar o inflamación de los tobillos o de las piernas o hinchazón facial.
- Inflamación del páncreas (pancreatitis). Contacte con su médico o con un servicio de urgencias si presenta dolor de estómago intenso y fiebre.
- Obstrucción intestinal (obstrucción del intestino). Contacte con su médico o con un servicio de urgencias si presenta dolor de estómago intenso, vómitos y estreñimiento.
- Reducción del flujo de bilis desde la vesícula biliar y/o inflamación de la vesícula biliar.
Contacte con su médico o con un servicio de urgencias si presenta color amarillento de la piel y la zona blanca de los ojos, picor, orina oscura y heces de color claro o si presenta dolor en el lado superior derecho o en la mitad de la zona del estómago.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- Desmayo. Si la frecuencia cardíaca y la respiración son normales y se despierta rápidamente, informe a su médico. En otros casos, solicite asistencia lo antes posible.
Otros efectos adversos incluidos:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- Infección del tracto respiratorio (cualquier infección en los senos nasales, la garganta, las vías respiratorias o los pulmones).
- Dolor de cabeza.
- Dolor de estómago, hinchazón del estómago, sensación de mareo (náuseas), inflamación del estoma (abertura artificial para la eliminación de desechos), vómitos.
- Enrojecimiento, dolor o inflamación en el lugar de la inyección.
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- Gripe o síntomas seudogripales.
- Disminución del apetito.
- Hinchazón de manos y/o pies.
- Problemas para dormir, ansiedad.
- Tos, dificultad para respirar.
- Pólipos (crecimiento de pequeñas masas anormales) en el intestino grueso.
- Gases (flatulencia).
- Estrechamiento o bloqueo del conducto del páncreas, que puede producir inflamación del
- páncreas.
- Inflamación de la vesícula biliar.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- Pólipos (crecimiento de pequeñas masas anormales) en el intestino delgado.
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- Reacción alérgica (hipersensibilidad).
- Retención de líquidos.
- Pólipos (crecimiento de pequeñas masas anormales) en el estómago.
Uso en niños y adolescentes
En general, los efectos adversos en niños y adolescentes son similares a los observados en adultos.
No se dispone de datos para los niños menores de 4 meses de edad.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Revestive
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja, el vial y la jeringa precargada después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar por debajo de 25ºC.
No congelar.
Tras la reconstitución, desde un punto de vista microbiológico, la solución debe usarse inmediatamente. Aun así, se ha demostrado estabilidad química y física durante 3 horas a 25ºC.
No utilice este medicamento si observa que la solución está turbia o contiene partículas.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente. Todas las agujas y jeringas deben desecharse en contenedores para objetos punzantes.
6. Contenido del envase e información adicional
Composición de Revestive
- El principio activo es teduglutida. Un vial de polvo contiene 5 mg de teduglutida. Tras la reconstitución, cada vial contiene 5 mg de teduglutida en 0,5 ml de disolución, que corresponde a una concentración de 10 mg/ml.
- Los demás componentes son L-histidina, manitol, fosfato de sodio monohidrato y fosfato disódico heptahidrato, hidróxido de sodio (ajuste de pH), ácido clorhídrico (ajuste de pH).
- El disolvente contiene agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Revestive es un polvo y un disolvente para solución inyectable (5 mg de teduglutida en vial, 0,5 ml de disolvente en jeringa precargada).
El polvo es blanco y el disolvente es transparente e incoloro.
Revestive se presenta en tamaños de envase de 1 vial de polvo con 1 jeringa precargada o 28 viales de polvo con 28 jeringas precargadas.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Takeda Pharmaceuticals International AG Ireland Branch
Block 2 Miesian Plaza
50 – 58 Baggot Street Lower
Dublin 2, D02 HW68
Irlanda
Fecha de la última revisión de este prospecto: 06/2023
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu. También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
Instrucciones para la preparación e inyección de Revestive
Información importante:
- Leer el prospecto antes de usar Revestive.
- Revestive se inyecta por debajo de la piel (vía subcutánea).
- No inyectar Revestive en una vena (vía intravenosa) ni en un músculo (vía intramuscular).
- Mantener Revestive fuera de la vista y del alcance de los niños.
- No usar Revestive después de la fecha de caducidad que aparece en la caja, el vial y la jeringa precargada. La fecha de caducidad es el último día del mes que se indica.
- Conservar por debajo de 25ºC.
- No congelar.
- Tras la reconstitución, desde un punto de vista microbiológico, la solución se debe usar inmediatamente. Sin embargo, se ha demostrado la estabilidad química y física durante 3 horas a 25ºC.
- No utilizar Revestive si la solución está turbia o contiene partículas.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no usa. De esta forma, ayudará a proteger el medio ambiente.
- Tirar todas las agujas y jeringas en un contenedor para objetos punzantes.
 Materiales incluidos en el envase:
Materiales incluidos en el envase:
- 1 o 28 viales con 5 mg de teduglutida en forma de polvo.
- 1 o 28 jeringas precargadas con disolvente.
Material necesario pero no incluido en el envase:
- Agujas para reconstitución (grosor 22G, largo 1½" [0,7 x 40 mm])
- Jeringas para inyección de 0,5 ml o 1 ml (con escala de intervalos de 0,02 ml o menos). Para losniños, se puede utilizar una jeringa para inyección de 0,5 ml (o más pequeña).
- Agujas finas para inyección subcutánea (p.ej., grosor 26G, largo 5/8" [0,45 x 16 mm] o agujas más pequeñas para niños, si procede).
- Toallitas impregnadas en alcohol.
- Hisopos de algodón impregnados en alcohol.
- Contenedor para objetos punzantes donde desechar las jeringas y agujas usadas de forma segura.
NOTA:Antes de comenzar, lávese las manos y asegúrese de que dispone de una superficie limpia donde manipular los materiales antes de proceder.
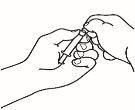
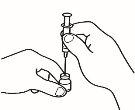
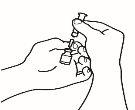
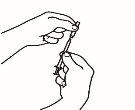
- Monte la jeringa precargada
Una vez preparado todo el material, debe montar la jeringa precargada. A continuación se muestra el procedimiento a seguir:
| 1.1 Coja la jeringa precargada con el disolvente y retire la parte superior del tapón de plástico blanco de la jeringa precargada de manera que pueda colocar la aguja de reconstitución. |
| 1.2 Acople la aguja de reconstitución (22G 1½" [0,7 x 40 mm]) en la jeringa precargada ya montada enroscando en el sentido de las agujas del reloj. |
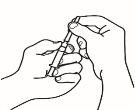
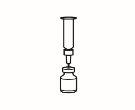
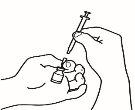
- Disuelva el polvo
Ahora ya puede disolver el polvo con el disolvente.
| 2.1 Retire el precinto verde del vial de polvo, limpie la parte superior con una toallita impregnada en alcohol y déjela secar. No toque la parte superior del vial. |
| 2.2 Retire el capuchón de la aguja de reconstitución de la jeringa precargada ya montada con el disolvente, sin tocar la punta de la aguja |
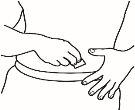
| 2.3 Coja el vial de polvo e introduzca la aguja de reconstitución acoplada a la jeringa precargada ya montada a través del centro del tapón de goma; a continuación, presione cuidadosamente el émbolo hasta el fondo, para inyectar todo el contenido del disolvente dentro del vial |
| 2.4 Deje la aguja de reconstitución y la jeringa vacía en el vial. Deje reposar el contenido durante aproximadamente 30 segundos. |
| 2.5 Haga rodar suavemente el vial entre las palmas de sus manos durante unos 15 segundos. A continuación, invierta el vial con cuidado una sola vez, con la aguja de reconstitución y la jeringa vacía aún en el vial. |
NOTA:No agite el vial. La agitación del vial puede producir espuma, que hace que sea difícil extraer la solución del vial.
| 2.6 Deje reposar el contenido del vial unos dos minutos. |
2.7 Observe si el vial contiene polvo sin disolver. Si aún queda polvo no disuelto, repita los pasos 2.5 y 2.6. No agite el vial. Si aún queda polvo sin disolver, deseche el vial y repita el procedimiento desde el principio con un nuevo vial.
NOTA:La disolución final debe ser transparente. Si la disolución está turbia o contiene partículas, no debe inyectarse.
NOTA:Una vez preparada, la solución debe usarse inmediatamente. Debe conservarse por debajo de 25ºC durante un máximo de tres horas.
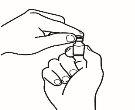
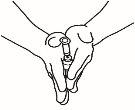
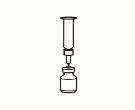
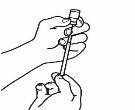
- Prepare la jeringa para inyección
| 3.1 Extraiga la jeringa de reconstitución de la aguja de reconstitución que aún está en el vial y deseche la jeringa de reconstitución. |
| 3.2 Coja la jeringa para inyección y acóplela a la aguja de reconstitución que aún está en el vial. |
| 3.3 Invierta el vial, de forma que quede boca abajo, deslice la punta de la aguja de reconstitución cerca del tapón y tire suavemente del émbolo para llenar la jeringa con el medicamento. |
NOTA:Si su médico le ha indicado que necesita dos viales, prepare una segunda jeringa precargada con disolvente y un segundo vial de polvo, tal y ycomo se muestra en los pasos 1 y 2. Con la misma jeringa que acaba de utilizar, extraiga la disolución del segundo vial repitiendo el paso 3.
| 3.4 Retire la jeringa para inyección de la aguja de reconstitución dejando la aguja en el vial. Tire el vial y la aguja de reconstitución al contenedor para objetos punzantes. |
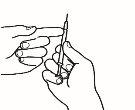
| 3.5 Coja la aguja de inyección, pero no retire el capuchón de plástico de la aguja. Acople la aguja a la jeringa para inyección que contiene el medicamento. |
| 3.6 Compruebe que no haya burbujas de aire. Si las hay, golpee suavemente con el dedo la jeringa hasta que las burbujas suban a la superficie. Seguidamente, presione el émbolo para expulsar las burbujas de aire. |
| 3.7 Su médico ha calculado su dosis en ml. Expulse el exceso de volumen de la jeringa con el capuchón de la aguja puesto hasta alcanzar el volumen de su dosis. |
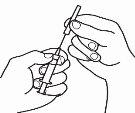
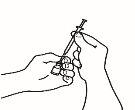
- Inyecte la solución
|
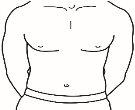
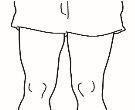
| 4.1 Localice una zona de su vientre, en la que le resulte fácil administrarse una inyección, o de su muslo si tiene dolor o endurecimiento del vientre (ver el diagrama). |
NOTA:No se aplique las inyecciones siempre en la misma zona, cambie de zona (utilice la parte superior, inferior, izquierda o derecha de su vientre) para evitar molestias. Evite las zonas inflamadas, hinchadas, con cicatrices o cubiertas por un lunar, una marca de nacimiento o cualquier tipo de lesión.
| 4.2 Una vez elegido el punto de inyección, limpie toda la zona con un hisopo de algodón impregnado en alcohol mediante movimientos circulares desde dentro hacia fuera. Deje secar la zona. |
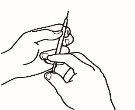
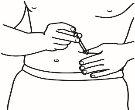
| 4.3 Retire el capuchón de plástico de la aguja que ha preparado para la inyección. Agarre suavemente la piel limpia del punto de inyección con una mano. Con la otra, sujete la jeringa como si fuera un lápiz. Flexione la muñeca hacia atrás e inserte rápidamente la aguja en un ángulo de 45º. |
4.4 Tire ligeramente del émbolo. Si observa sangre en la jeringa, retire la aguja y sustitúyala en la jeringa para inyección por otra nueva y limpia del mismo tamaño. Aún puede utilizar el medicamento que se encuentra dentro de la jeringa. Trate de escoger otro punto de inyección en la misma zona de piel limpia.
4.5 Inyecte el medicamento despacio, presionando el émbolo de forma continuada, hasta que haya inyectado todo el medicamento y la jeringa esté vacía.
4.6 Extraiga rápidamente la aguja de la piel y tire la aguja y la jeringa al contenedor para objetos punzantes. Es posible que sangre un poco. Si fuera necesario, presione con suavidad la zona de inyección con un hisopo de algodón impregnado en alcohol o una gasa 2x2 hasta que cese la hemorragia.
4.7 Deseche todas las agujas y jeringas en un contenedor para objetos punzantes o de paredes gruesas (por ejemplo, un bote de detergente con tapón). Debe utilizar un contenedor para objetos punzantes o de paredes gruesas (incluida la tapa y las paredes laterales). Si necesita un contenedor para objetos punzantes, por favor consulte a su médico.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a REVESTIVE 5 MG POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: COMPRIMIDO, DesconocidaPrincipio activo: Fenilbutirato sodioFabricante: Immedica Pharma AbRequiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, DesconocidaPrincipio activo: Fenilbutirato sodioFabricante: Immedica Pharma AbRequiere recetaForma farmacéutica: CAPSULA, 84 MG de eliglustat (como tartrato)Principio activo: EliglustatFabricante: Sanofi B.V.Requiere receta
Médicos online para REVESTIVE 5 MG POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de REVESTIVE 5 MG POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes