
RETACRIT 5000 IU/0.5 ml SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE

How to use RETACRIT 5000 IU/0.5 ml SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Retacrit 1,000UI/0.3ml solution for injection in a pre-filled syringe
Retacrit 2,000UI/0.6ml solution for injection in a pre-filled syringe
Retacrit 3,000UI/0.9ml solution for injection in a pre-filled syringe
Retacrit 4,000UI/0.4ml solution for injection in a pre-filled syringe
Retacrit 5,000UI/0.5ml solution for injection in a pre-filled syringe
Retacrit 6,000UI/0.6ml solution for injection in a pre-filled syringe
Retacrit 8,000UI/0.8ml solution for injection in a pre-filled syringe
Retacrit 10,000UI/1ml solution for injection in a pre-filled syringe
Retacrit 20,000UI/0.5ml solution for injection in a pre-filled syringe
Retacrit 30,000UI/0.75ml solution for injection in a pre-filled syringe
Retacrit 40,000UI/1ml solution for injection in a pre-filled syringe
epoetin zeta
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Retacrit and what is it used for
- What you need to know before you use Retacrit
- How to use Retacrit
- Possible side effects
- Storing Retacrit
- Contents of the pack and other information
1. What is Retacrit and what is it used for
Retacrit contains the active substance epoetin zeta, a protein that stimulates the bone marrow to produce more red blood cells, cells that carry hemoglobin (the substance that carries oxygen). Epoetin zeta is a copy of the human protein erythropoietin and works in the same way.
- Retacrit is indicated for the treatment of symptomatic anemia caused by a renal disease
- in children undergoing hemodialysis;
- in adults undergoing hemodialysis or peritoneal dialysis;
- in adults with severe anemia who have not yet undergone dialysis.
If you have kidney disease, you may have a low number of red blood cells if your kidney does not produce enough erythropoietin (necessary for the production of red blood cells). Retacrit is prescribed to stimulate the bone marrow to produce more red blood cells.
- Retacrit is indicated for the treatment of anemia in adults receiving chemotherapy for the treatment of solid tumors, malignant lymphoma, or multiple myeloma (bone marrow cancer) who may need a blood transfusion. Retacrit may reduce the need for a blood transfusion in these patients.
- Retacrit is indicated in adults with moderate anemia who are about to donate blood beforeundergoing surgery, so that it can be readministered during or after the surgical procedure. Since Retacrit stimulates the production of red blood cells, doctors can withdraw more blood from these individuals.
- Retacrit is indicated in adults with moderate anemia who are about to undergo major scheduled orthopedic surgery(e.g., hip or knee replacement operations)to reduce the potential need for blood transfusion.
- Retacrit is indicated in adult patients with anemia with a bone marrow disorder that causes a severe alteration in the creation of blood cells (myelodysplastic syndromes).Retacrit may reduce the need for a blood transfusion.
2. What you need to know before you use Retacrit
Do not use Retacrit
- If you are allergicto epoetin zeta or any of the other ingredients of this medicine (listed in section 6).
- If you have been diagnosed with pure red cell aplasia(the bone marrow cannot produce enough red blood cells) after previous treatment with any product that stimulates the production of red blood cells (including Retacrit). See section 4.
- If you have uncontrolled high blood pressure.
- To stimulate the production of red blood cells (so that your doctors can withdraw more blood) if you cannot receive transfusions of your own bloodduring or after surgery.
- If you are about to undergo major scheduled orthopedic surgery (such as hip or knee replacement) and you:
- have severe heart disease;
- have severe vein or artery problems;
- have recently had a heart attack or stroke;
- cannot take medicines to thin your blood.
You may not be suitable for Retacrit. Talk to your doctor. Some people need medicines to reduce the risk of blood clots during treatment with Retacrit. If you cannot take medicines to prevent blood clots, you should not take Retacrit.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Retacrit.
Be careful with Retacrit
Retacrit and other products that stimulate the production of red blood cells may increase the risk of blood clots in all patients.This risk may be greater if you have other risk factors (e.g., if you have had a blood clot in the past or have overweight, diabetes, heart disease, or are bedridden for a long time due to surgery or illness). Tell your doctor about any of these things. Your doctor will help you decide if Retacrit is suitable for you.
Talk to your doctorif you identify with any of the following situations .You may still be able to use Retacrit, but discuss it with your doctor first.
- If you know you haveor have had:
- high blood pressure;
- seizures or convulsions;
- liver disease;
- anemia from other causes;
- porphyria (a rare blood disorder).
- If you are a patient with chronic kidney disease, and especially if you do not respond well to Retacrit, your doctor will check your Retacrit dose, because repeatedly increasing the dose of Retacrit if you do not respond to treatment may increase the risk of heart or blood vessel problems and may increase the risk of heart attack, stroke, and death.
- If you are a cancer patient, you should know that products that stimulate the production of red blood cells (such as Retacrit) may act as a growth factor and, therefore, may affect, in theory, the progression of cancer. Depending on your individual situation, a blood transfusion may be preferable. Discuss this with your doctor.
- If you are a cancer patient, you should know that the use of Retacrit may be associated with lower survival and higher mortality rates in patients with head and neck cancer and metastatic breast cancer receiving chemotherapy.
- Severe skin reactions such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN)have been observed with the administration of epoetins.
SJS/TEN can initially appear as red spots like targets or circular patches, often with central blisters on the trunk. Ulcers in the mouth, throat, nose, genitals, and eyes (eye irritation and swelling) may also appear. These severe skin rashes are often preceded by fever or flu-like symptoms. The rashes can progress to widespread skin peeling and potentially life-threatening complications.
If you develop a severe skin rash or any of these other skin symptoms, stop taking Retacrit and contact your doctor or seek medical attention immediately.
Be careful with other products that stimulate the production of red blood cells
Retacrit belongs to one of the groups of products that stimulate the production of red blood cells, like the human protein erythropoietin. Your doctor should record the exact name of the product you are using.
If, during your treatment, you are given a product from this group, different from Retacrit, talk to your doctor or pharmacist before using it.
Other medicines and Retacrit
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
If you are using Retacrit with a medicine called cyclosporin(e.g., after a kidney transplant), your doctor may request blood tests to check the level of cyclosporin.
Iron supplements and other blood stimulantsmay increase the effectiveness of Retacrit. Your doctor will decide if you should use them.
If you visit a hospital, clinic, or general practitioner, tell them that you are being treated with Retacrit, as it may affect other treatments or test results.
Pregnancy, breastfeeding, and fertility
It is important that you inform your doctorif you identify with any of the following situations .You may still be able to use Retacrit, but discuss it with your doctor first.
- If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
- If you are breastfeeding.
No data are available on the effects of epoetin zeta on fertility.
Driving and using machines
Retacrit has no or negligible influence on the ability to drive and use machines.
Retacrit contains phenylalanine
Retacrit contains 0.5 mg of phenylalanine per ml.
Phenylalanine may be harmful if you have phenylketonuria (PKU), a rare genetic disorder in which phenylalanine accumulates because the body cannot eliminate it properly (see section).
Retacrit contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose, i.e., it is essentially "sodium-free".
3. How to use Retacrit
Follow exactly the administration instructions of this medication as indicated by your doctor.In case of doubt, consult your doctor again.
Your doctor has performed blood testsand has decided that you need Retacrit.
Retacrit can be administered by injection:
- Eitherin a vein or through a tube that is inserted into a vein (intravenously).
- Orunder the skin (subcutaneously).
Your doctor will decide how Retacrit will be injected. The injections will usually be performed by a doctor, nurse, or other healthcare professional. Later on, and depending on the reason why you need treatment with Retacrit, some people may learn to inject the medication under the skin: see Instructions on how to inject Retacrit.
Retacrit should not be used:
- after the expiration date on the label and outer carton;
- if you know or believe it may have been accidentally frozen, or
- if there has been a malfunction in the refrigerator.
The dose of Retacrit you receive is based on your body weight in kilograms. The cause of your anemia is also a factor that your doctor will take into account when deciding the correct dose.
Your doctor will check your blood pressureperiodically while you are being treated with Retacrit.
Patients with renal insufficiency
- Your doctor will keep your hemoglobin level between 10 and 12 g/dl, as a high hemoglobin level can increase the risk of blood clots and death. In children, the hemoglobin level should be kept between 9.5 and 11 g/dl.
- The usual starting doseof Retacrit for adults and children is 50 IU/kg (international units per kilogram) three times a week.
- In patients on peritoneal dialysis, Retacrit can be administered twice a week.
- In both adults and children, Retacrit is administered by injection, either in a vein (intravenously) or through a tube inserted into a vein. When this access cannot be easily achieved (through a vein or tube), your doctor may decide that Retacrit should be injected under the skin (subcutaneously). This includes patients on dialysis and those who are not yet on dialysis.
- Your doctor will request periodic blood tests to see how your anemia is responding and may adjust the dose, usually no more frequently than every four weeks. An increase in hemoglobin of more than 2 g/dl during a four-week period should be avoided.
- Once the anemia has been corrected, your doctor will continue to perform periodic blood tests. You may need to have the dose and frequency of administration of Retacrit adjusted to maintain your response to treatment. Your doctor will use the minimum effective dose to control the symptoms of anemia.
- If you do not respond well to Retacrit, your doctor will check your dose and inform you if you need to modify the dose of Retacrit.
- If you receive a dosing interval greater than once a week of Retacrit, you may not maintain adequate hemoglobin levels and may require an increase in the dose of Retacrit or the frequency of administration.
- You may be given iron supplements before and during treatment with Retacrit to increase its effectiveness.
- If you are undergoing dialysis at the time of starting treatment with Retacrit, your dialysis schedule may need to be adjusted. Your doctor will decide if this is necessary.
Adults on chemotherapy
- Your doctor may start treatment with Retacrit if your hemoglobin is equal to or less than 10 g/dl.
- Your doctor will keep your hemoglobin concentration between 10 and 12 g/dl, as a high hemoglobin concentration can increase the risk of blood clots and death.
- The starting dose is 150 IU/kg of body weight three times a week or 450 IU/kg of body weight once a week.
- Retacrit is administered by injection under the skin.
- Your doctor will request blood tests and may adjust the dose, depending on how your anemia responds to treatment with Retacrit.
- You may be given iron supplements before and during treatment with Retacrit to increase its effectiveness.
- Normally, you will continue to receive treatment with Retacrit for one month after completing chemotherapy.
Adults donating their own blood
- The usual doseis 600 IU/kg of body weight twice a week.
- Retacrit is administered by injection into a vein immediately after donating blood for three weeks before surgery.
- You may be given iron supplements before and during treatment with Retacrit to increase its effectiveness.
Adults scheduled for major orthopedic surgery
- The recommended doseis 600 IU/kg of body weight once a week.
- Retacrit is administered by injection under the skin for three weeks before surgery and on the day of surgery.
- In cases where the pre-operative time needs to be reduced, a daily dose of 300 IU/kg will be administered for a maximum of 10 days before surgery, on the day of surgery, and for four days immediately after.
- If blood tests show a hemoglobin level that is too high before surgery, treatment will be discontinued.
- You may be given iron supplements before and during treatment with Retacrit to increase its effectiveness.
Adults with myelodysplastic syndrome
- Your doctor may start treatment with Retacrit if your hemoglobin concentration is equal to or less than 10 g/dl. The goal of treatment is to maintain the hemoglobin level between 10 and 12 g/dl, as a higher hemoglobin level can increase the risk of blood clots and death.
- Retacrit is administered by injection under the skin.
- The starting dose is 450 IU per kilogram of body weight once a week.
- Your doctor will request blood tests and may adjust the dose, depending on how your anemia responds to treatment with Retacrit.
Instructions on how to inject Retacrit
When starting treatment, medical personnel usually inject Retacrit. Later on, your doctor may suggest that you or your caregiver learn to inject Retacrit under the skin (subcutaneously) by yourself.
- Do not attempt to inject yourself unless your doctor or nurse has shown you how to do it.
- Follow exactly the administration instructions of Retacrit as indicated by your doctor or nurse.
- Only use Retacrit if it has been stored correctly; see section5,Storage of Retacrit.
- Before use, let the Retacrit syringe stand at room temperature until it reaches room temperature. This usually takes between 15 and 30minutes.
Use only one dose of Retacrit per syringe.
If Retacrit is injected under the skin (subcutaneously), the amount injected is not normally more than 1 milliliter (1 ml) in a single injection. For larger volumes, more than one injection site should be chosen.
Retacrit is administered alone and not mixed with other injectable liquids.
Do not shake Retacrit syringes.Prolonged vigorous shaking can damage the product. If the product has been shaken forcefully, do not use it.
How to inject yourself using a pre-filled syringe
- Remove the packaging with the pre-filled syringe from the refrigerator.
- Remove the blister with the pre-filled syringe from the packaging. When the packaging contains blisters with more than one pre-filled syringe, cut the blister with a pre-filled syringe along the perforated part, return the rest of the blisters with pre-filled syringes to the packaging, and return the packaging to the refrigerator.
- Open the blister with the pre-filled syringe after removing it from the refrigerator. The liquid should reach room temperature. Do notremove the needle cover from the syringe while letting the pre-filled syringe reach room temperature.

- Check the syringe to ensure it is the correct dose, has not expired, is not damaged, and the liquid is clear and not frozen.
- Do not use the pre-filled syringe if:
- The packaging is open or damaged.
- The medication is cloudy or has changed color or the liquid has particles floating in it.
- Any part of the pre-filled syringe appears cracked or broken or liquid has leaked from the syringe.
- The pre-filled syringe has been dropped. The pre-filled syringe may be broken even if you cannot see the break.
- The needle cover is missing or not properly placed.
- The expiration date printed on the label has passed.
In all the above cases, discard the pre-filled syringe and use a new pre-filled syringe.
- Choose an injection site. Good sites are the top of the thigh and around the belly (abdomen) but away from the navel. Vary the site from day to day.
- Wash your hands. Use an antiseptic swab on the injection site to disinfect it.
- Hold the pre-filled syringe by the syringe body with the needle cover pointing upwards.
- Do nothold it by the plunger, plunger head, or needle cover.
- Do notpull the plunger at any time.
- Do notremove the needle cover from the syringe until you are ready to inject your medication.
- Remove the needle cover from the syringe by holding the syringe body and pulling the needle cover carefully, without twisting. Discard the needle cover. Do notreplace the needle cover. Do notpush the plunger, touch the needle, or shake the syringe.
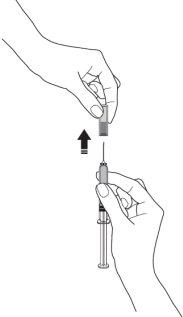
- Pinch a skin fold between your thumb and index finger. Do notcompress it.
- With your other hand, hold the pre-filled syringe like a pencil. Use a quick "darting" motion to insert the needle into the skin at an approximate angle of 45 degrees.
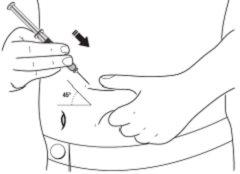
- Insert the needle fully. Your doctor or nurse has shown you how to do this.
- Push the plunger with your thumb as far as you can to inject all of the liquid. Push slowly and steadily, keeping the skin fold pinched.
- When the plunger has reached the end, remove the needle and release the skin.
- When the needle is removed from the skin, there may be a little bleeding at the injection site. This is normal. You can press an antiseptic swab over the injection site for a few seconds after the injection.
- Do notattempt to replace the needle cover. Dispose of the used syringe in a puncture-proof container.
- Never put used syringes in the normal household trash.
How to inject yourself using a pre-filled syringe with a needle protection mechanism
Your pre-filled syringe has a needle protection mechanism attached to protect you from a needle stick injury.
- Remove the packaging with the pre-filled syringe with a needle protection mechanism from the refrigerator.
- Remove the blister with the pre-filled syringe from the packaging. When the packaging contains blisters with more than one pre-filled syringe, cut the blister with a pre-filled syringe along the perforated part, return the rest of the blisters with pre-filled syringes to the packaging, and return the packaging to the refrigerator.
- Open the blister with the pre-filled syringe by removing the blister cap.
- Remove the pre-filled syringe from the blister by holding the syringe body.
- Do nothold the gray needle cover or the plunger.
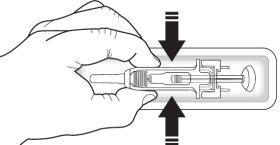
- Check the syringe to ensure the needle protection mechanism covers the syringe body. Do notpush the needle protection mechanism over the needle cover before injection. This may activate or block the needle protection mechanism. If the needle protection mechanism covers the needle, it means it has been activated.
- The liquid should reach room temperature. Do notremove the needle cover from the syringe while letting the pre-filled syringe reach room temperature.
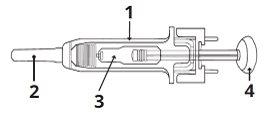
1 | Needle protection mechanism |
2 | Needle cover |
3 | Medication |
4 | Plunger |
- Check the syringe to ensure it is the correct dose, has not expired, is not damaged, and the liquid is clear and not frozen.
- Do not use the pre-filled syringe if:
- The cardboard packaging is open or damaged.
- The needle protection mechanism is missing, has come off, or has been activated.
- The medication is cloudy or has changed color or the liquid has particles floating in it. Do notinspect the product through the plastic of the safety device.
- Any part of the pre-filled syringe appears cracked or broken or liquid has leaked from the syringe.
- The pre-filled syringe has been dropped. The pre-filled syringe may be broken even if you cannot see the break.
- The needle cover is missing or not properly placed.
- The expiration date printed on the label has passed.
In all the above cases, discard the pre-filled syringe and use a new pre-filled syringe.
- Choose an injection site. Good sites are the top of the thigh and around the belly (abdomen) but away from the navel. Vary the site from day to day.
- Wash your hands. Use an antiseptic swab on the injection site to disinfect it.
- Hold the pre-filled syringe by the needle protection mechanism body with the needle cover pointing upwards.
- Do nothold it by the plunger head, plunger, or needle cover.
- Do notpull the plunger at any time.
- Do notremove the needle cover from the pre-filled syringe until you are ready to inject your medication.
- Remove the needle cover from the syringe by holding the syringe body and pulling the needle cover carefully, without twisting. Discard the needle cover. Do notreplace the needle cover. Do notpush the plunger, touch the needle, or shake the syringe.
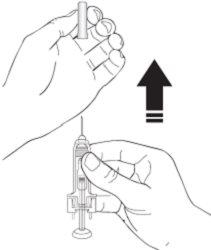
- Pinch a skin fold between your thumb and index finger. Do notcompress it.
- With your other hand, hold the pre-filled syringe like a pencil. Use a quick "darting" motion to insert the needle into the skin at an approximate angle of 45 degrees.
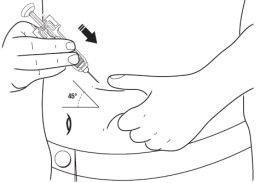
- Insert the needle fully. Your doctor or nurse has shown you how to do this.
- Press the plunger while holding the collar with your fingers until the complete dose has been administered. The needle protection mechanism will NOT be activated unless the dose has been administered in its entirety.
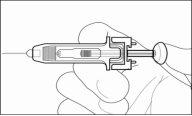
- When the plunger has reached the end, remove the needle and release the skin.
- Release the plunger and allow the syringe to move upwards until the entire needle is guarded and locked in place.
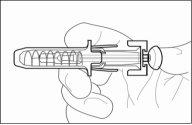
- When the needle is removed from the skin, there may be a little bleeding at the injection site. This is normal. You can press an antiseptic swab over the injection site for a few seconds after the injection.
- Do notattempt to replace the needle cover. Dispose of the used syringe in a puncture-proof container.
- Never put used syringes in the normal household trash.
How to inject yourself using a pre-filled syringe with a needle trap (needle safety device)
Your syringe has a needle trap (needle safety device) attached, which is designed to help prevent accidental injuries after the correct administration of injectable medications. It consists of a plastic device that blocks the needle and is firmly attached to the syringe label. Together, these two components perform the function of a needle trap (safety device).
The plastic blocking device attached to the syringe label requires specific actions from the user to "activate" it, making the needle harmless after administering the injection:
- Remove the packaging with the pre-filled syringe from the refrigerator.
- Remove the blister with the pre-filled syringe from the packaging. When the packaging contains blisters with more than one pre-filled syringe, cut the blister with a pre-filled syringe along the perforated part, return the rest of the blisters with pre-filled syringes to the packaging, and return the packaging to the refrigerator.
- Remove the pre-filled syringe from the packaging.
... (rest of the translation remains the same)
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor or nurse immediatelyif you experience any of the effects mentioned in the following list.
Severe skin rashes, such as Stevens-Johnson syndrome and toxic epidermal necrolysis, have been observed with the administration of epoetins. These reactions can appear as red spots or patches, often with central blisters on the trunk, skin peeling, and ulcers in the mouth, throat, nose, genitals, and eyes, and may be preceded by fever and flu-like symptoms. Stop using Retacrit if you experience these symptoms and contact your doctor or seek medical attention immediately. See also section 2.
Very common: may affect more than 1 in 10 people.
- Diarrhea
- Stomach upset
- Vomiting
- Fever
- In patients with kidney failure who have not yet undergone dialysis, respiratory tract congestion, such as nasal congestion and sore throat, have been reported.
Common: may affect up to 1 in 10 people.
- Increased blood pressure. Headaches, especially sudden, sharp, and migraine-like, feeling of confusion or seizuresmay be signs of a sudden increase in blood pressure. This requires urgent treatment. This increase may require treatment with medications (or adjustment of the dosage of medications you are already taking for high blood pressure).
- Blood clots(including deep vein thrombosis and embolism) that may require urgent treatment. You may experience chest pain, difficulty breathing, and painful swelling and redness, usually in one leg.
- Cough.
- Itching of the skin, which can result from an allergic reaction.
- Bone or muscle pain
- Flu-like symptoms, such as headache, stinging, and pain in the joints, feeling of weakness, chills, fatigue, and dizziness. These may be more frequent at the start of treatment. If you experience these symptoms during intravenous injection, a slower administration of the injection may help prevent them from occurring again.
- Redness, burning, and pain at the injection site
- Swelling of the ankles, feet, or toes
- Pain in the arm or leg
Uncommon: may affect up to 1 in 100 people.
- High levels of potassium in the bloodthat can cause an abnormal heart rhythm (this is a very common side effect in patients on dialysis).
- Tremors
- Nasal or airway congestion
- Allergic reaction
- Hives
Rare: may affect up to 1 in 1,000 people.
- Symptoms of pure red cell aplasia (PRCA).
PRCA is the inability to produce enough red blood cells in the bone marrow. PRCA can cause sudden and severe anemia. The symptoms are:
- Unusual fatigue,
- Feeling of dizziness,
- Difficulty breathing.
Very rare cases of PRCA have been reported, mainly in patients with kidney disease after months or years of treatment with Retacrit and other products that stimulate the production of red blood cells.
- There may be an increase in the number of small blood cells (called platelets) that normally participate in the formation of blood clots, especially when treatment is started. Your doctor will check this.
- Severe allergic reactions that can include:
- swollen face, lips, mouth, tongue, or throat;
- difficulty swallowing or breathing;
- itchy rash (hives).
- A disorder that affects the blood that can cause pain, dark urine, or increased sensitivity of the skin to sunlight (porphyria).
If you are on hemodialysis:
- Blood clots(thrombosis) can form in the dialysis fistula. This is more common if you have low blood pressure or if your fistula has complications.
- Blood clotscan also form in your hemodialysis system. Your doctor may decide to increase your heparin dose during dialysis.
If you experience any of these effects or if you notice any other effect while being treated with Retacrit, tell your doctor or nurse immediately.
Reporting of side effects
If you experience any side effect, consult your doctor, pharmacist, or nurse, even if it is a possible side effect that does not appear in this leaflet. You can also report them directly through the Spanish Medicines Vigilance System: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Retacrit
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the box and label after "EXP". The expiration date is the last day of the month indicated.
Store in a refrigerator (2°C-8°C). You can take Retacrit out of the refrigerator and keep it at room temperature (up to 25°C) for a maximum of 3 days. Once a syringe has been removed from the refrigerator and has reached room temperature (up to 25°C), it must be used within 3 days or discarded.
Do not freeze or shake.
Keep it in the outer packaging to protect it from light.
Do not use this medicine if you notice that the seal is broken or if the liquid has a color or particles can be seen floating in it. If you notice any of these situations, discard the medicine.
Medicines should not be disposed of through wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package contents and additional information
Composition of Retacrit
The active substance is epoetin zeta (produced using recombinant DNA technology in Chinese hamster ovary cell lines).
Retacrit 1,000 UI/0.3 ml solution for injection in pre-filled syringe
1 pre-filled syringe with 0.3 ml of solution for injection contains 1,000 international units (IU) of epoetin zeta (recombinant human erythropoietin). The solution contains 3,333 IU of epoetin zeta per ml.
Retacrit 2,000 UI/0.6 ml solution for injection in pre-filled syringe
1 pre-filled syringe with 0.6 ml of solution for injection contains 2,000 international units (IU) of epoetin zeta (recombinant human erythropoietin). The solution contains 3,333 IU of epoetin zeta per ml.
Retacrit 3,000 UI/0.9 ml solution for injection in pre-filled syringe
1 pre-filled syringe with 0.9 ml of solution for injection contains 3,000 international units (IU) of epoetin zeta (recombinant human erythropoietin). The solution contains 3,333 IU of epoetin zeta per ml.
Retacrit 4,000 UI/0.4 ml solution for injection in pre-filled syringe
1 pre-filled syringe with 0.4 ml of solution for injection contains 4,000 international units (IU) of epoetin zeta (recombinant human erythropoietin). The solution contains 10,000 IU of epoetin zeta per ml.
Retacrit 5,000 UI/0.5 ml solution for injection in pre-filled syringe
1 pre-filled syringe with 0.5 ml of solution for injection contains 5,000 international units (IU) of epoetin zeta (recombinant human erythropoietin). The solution contains 10,000 IU of epoetin zeta per ml.
Retacrit 6,000 UI/0.6 ml solution for injection in pre-filled syringe
1 pre-filled syringe with 0.6 ml of solution for injection contains 6,000 international units (IU) of epoetin zeta (recombinant human erythropoietin). The solution contains 10,000 IU of epoetin zeta per ml.
Retacrit 8,000 UI/0.8 ml solution for injection in pre-filled syringe
1 pre-filled syringe with 0.8 ml of solution for injection contains 8,000 international units (IU) of epoetin zeta (recombinant human erythropoietin). The solution contains 10,000 IU of epoetin zeta per ml.
Retacrit 10,000 UI/1 ml solution for injection in pre-filled syringe
1 pre-filled syringe with 1 ml of solution for injection contains 10,000 international units (IU) of epoetin zeta (recombinant human erythropoietin). The solution contains 10,000 IU of epoetin zeta per ml.
Retacrit 20,000 UI/0.5 ml solution for injection in pre-filled syringe
1 pre-filled syringe with 0.5 ml of solution for injection contains 20,000 international units (IU) of epoetin zeta (recombinant human erythropoietin). The solution contains 40,000 IU of epoetin zeta per ml.
Retacrit 30,000 UI/0.75 ml solution for injection in pre-filled syringe
1 pre-filled syringe with 0.75 ml of solution for injection contains 30,000 international units (IU) of epoetin zeta (recombinant human erythropoietin). The solution contains 40,000 IU of epoetin zeta per ml.
Retacrit 40,000 UI/1 ml solution for injection in pre-filled syringe
1 pre-filled syringe with 1 ml of solution for injection contains 40,000 international units (IU) of epoetin zeta (recombinant human erythropoietin). The solution contains 40,000 IU of epoetin zeta per ml.
The other ingredients are sodium dihydrogen phosphate dihydrate, disodium phosphate dihydrate, sodium chloride (see section 2 "Retacrit contains sodium"), calcium chloride dihydrate, polysorbate 20, glycine, leucine, isoleucine, threonine, glutamic acid, phenylalanine (see section 2 "Retacrit contains phenylalanine"), water for injections, sodium hydroxide (for pH adjustment), hydrochloric acid (for pH adjustment).
Appearance and package contents
Retacrit is a clear and colorless solution for injection presented in transparent glass syringes with a fixed injection needle.
The pre-filled syringes contain between 0.3 and 1 ml of solution, depending on the epoetin zeta content (see "Composition of Retacrit").
Each pack includes 1, 4, or 6 pre-filled syringes with or without a safety needle protector or with a plastic blocker attached to the syringe label.
Multidose packs contain 4 (4 packs of 1) or 6 (6 packs of 1) pre-filled syringes.
Marketing authorization holder
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Brussels
Belgium
Manufacturers
Hospira Zagreb d.o.o.
Prudnicka cesta 60
10291 Prigorje Brdovecko
Croatia
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Spain
Pfizer, S.L.
Tel: +34 91 490 99 00
Date of last revision of this leaflet: 07/2023.
Detailed information about this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RETACRIT 5000 IU/0.5 ml SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 20000 IUActive substance: erythropoietinManufacturer: Sandoz GmbhPrescription requiredDosage form: INJECTABLE, 20,000 IUActive substance: erythropoietinManufacturer: Sandoz GmbhPrescription requiredDosage form: INJECTABLE, 40,000 IU/ml of epoetin alfaActive substance: erythropoietinManufacturer: Sandoz GmbhPrescription required
Online doctors for RETACRIT 5000 IU/0.5 ml SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about RETACRIT 5000 IU/0.5 ml SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















