
QUINSAIR 240 MG SOLUCION PARA INHALACION POR NEBULIZADOR

Cómo usar QUINSAIR 240 MG SOLUCION PARA INHALACION POR NEBULIZADOR
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Quinsair 240mg solución para inhalación por nebulizador
levofloxacino
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Quinsair y para qué se utiliza
- Qué necesita saber antes de empezar a usar Quinsair
- Cómo usar Quinsair
- Posibles efectos adversos
- Conservación de Quinsair
- Contenido del envase e información adicional
1. Qué es Quinsair y para qué se utiliza
Quinsair contiene un medicamento antibiótico denominado levofloxacino. Pertenece al grupo de antibióticos llamados fluoroquinolonas.
Quinsair se utiliza para tratar infecciones de pulmóncausadas por Pseudomonasaeruginosaen adultos con fibrosis quística. Es un antibiótico que se aspira (inhala) directamente a los pulmones, donde mata a las bacterias causantes de la infección. Esto ayuda a mejorar la respiración en las personas con fibrosis quística.
2. Qué necesita saber antes de empezar a usar Quinsair
No use Quinsair:
- si es alérgicoa levofloxacino,o a cualquier otro antibiótico quinolónico, como moxifloxacino, ciprofloxacino u ofloxacino, o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6)
- si ha tenido alguna vez problemas con sus tendones (inflamacióno rotura de un tendón) durante el tratamiento con un antibiótico quinolónico o fluoroquinolónico
- si padece de epilepsia
- si está embarazadao en periodo de lactancia
Advertencias y precauciones
Antes de empezar a tomar este medicamento
No debe tomar medicamentos antibacterianos que contengan fluoroquinolonas o quinolonas, incluido Quinsair, si ha experimentado alguna reacción adversa grave con anterioridad al tomar una quinolona o una fluoroquinolona. Si este es su caso, debe informar a su médico lo antes posible.
Durante el tratamiento con este medicamento
En raras ocasiones pueden aparecer dolor e hinchazón en las articulaciones e inflamación o rotura de los tendones. El riesgo es mayor si es usted una persona de edad avanzada (mayor de 60 años), ha recibido un trasplante de un órgano, tiene problemas de riñón o está en tratamiento con corticoesteroides. La inflamación y la rotura de tendones se puede producir en las primeras 48 horas de tratamiento e incluso hasta varios meses después de interrumpir el tratamiento con Quinsair. Al primer signo de dolor o inflamación de un tendón (por ejemplo, en el tobillo, la muñeca, el codo, el hombro o la rodilla), deje de tomar Quinsair, póngase en contacto con su médico y mantenga en reposo la zona dolorosa. Evite cualquier ejercicio innecesario, ya que podría aumentar el riesgo de rotura de un tendón.
Informe a su médico antes de empezar a usar Quinsair
si padece o ha padecido alguna de las dolencias siguientes:
- Efectos adversos graves de duración prolongada, incapacitantes y potencialmente irreversibles
Los medicamentos antibacterianos que contienen fluoroquinolonas o quinolonas, incluido Quinsair, se han asociado a efectos adversos muy raros pero graves, algunos de los cuales fueron de larga duración (persistentes durante meses o años), incapacitantes o potencialmente irreversibles. Esto incluye dolor en los tendones, los músculos y las articulaciones de las extremidades superiores e inferiores, dificultad para caminar, sensaciones anómalas como pinchazos, hormigueo, cosquilleo, entumecimiento, quemazón o escozor (parestesia), trastornos sensitivos tales como disminución de la vista, del gusto, del olfato y la audición, depresión, disminución de la memoria y de la concentración, cansancio intenso y trastornos del sueño graves.
Si experimenta cualquiera de estos efectos adversos después de tomar Quinsair, póngase en contacto de forma inmediata con su médico antes de continuar con el tratamiento. Usted y su médico decidirán si continuar o no el tratamiento, considerando también el uso de un antibiótico de otra clase.
- Problemas graves en el riñón.
- Una reacción alérgica grave. Los síntomas se incluyen en la sección 4.
- Reacciones cutáneas graves
Si recibe tratamiento con Quinsair, puede sufrir una reacción cutánea grave como formación de ampollas o lesiones. Informe a su médico si nota cualquier reacción cutánea después de usar Quinsair.
- Problemas en el hígado. Los síntomas se incluyen en la sección 4,
- Alteraciones del ritmo cardiaco
Quinsair puede causar cambios en su ritmo cardiaco, especialmente si está tomando medicamentos para tratar problemas cardiacos o los niveles bajos de potasio o de magnesio en sangre. Las mujeres que toman estos medicamentos es más probable que se vean afectadas. Si experimenta palpitaciones o latido cardiaco irregular mientras esté usando Quinsair, debe informar a su médico inmediatamente.
- Ataques y convulsiones
Los antibióticos quinolónicos, incluido Quinsair, pueden provocar ataques o convulsiones. Si esto ocurre, deje de usar Quinsair y póngase inmediatamente en contacto con su médico.
- Depresión o problemas de salud mental.
- Lesión en los nervios
En raras ocasiones, puede experimentar síntomas de lesión en los nervios (neuropatía), como dolor, quemazón, escozor, hormigueo, entumecimiento y/o debilidad, en especial en pies y piernas o manos y brazos. Si esto sucede, deje de tomar el tratamiento con Quinsair e informe de forma inmediata a su médico para prevenir el desarrollo de un trastorno potencialmente irreversible.
- Enfermedad que provoca debilidad muscular y fatiga llamada miastenia gravis.
- Inflamación de un tendón que cause dolor, rigidez y/o hinchazón en las articulaciones (tendinitis)
- Si después de recibir Quinsair ha experimentado dificultad para respirar, que puede ser leve o grave (broncoespasmo)
- Expectoración o mucosidad con sangre procedentes de las vías respiratorias
- Deficiencia de glucosa-6-fosfato deshidrogenasa
En los pacientes con deficiencia de glucosa-6-fosfato deshidrogenasa (una enfermedad hereditaria rara) los antibióticos quinolónicos, como Quinsair, pueden causar predisposición a complicaciones de la sangre (hematológicas) que den lugar a un aumento repentino de la temperatura corporal, coloración amarillenta de la piel y las membranas mucosas, orina de color oscuro, palidez, cansancio, respiración rápida y pesada y pulso rápido y débil. Consulte a su médico en caso de duda.
- Diabetes
Los antibióticos quinolónicos, incluido Quinsair, pueden provocar unos niveles de glucosa en sangre demasiado altos o demasiado bajos. Si es usted diabético, debe controlar cuidadosamente sus niveles de glucosa en sangre.
- Diarrea
Puede padecer diarrea durante o después del tratamiento con Quinsair. Si la diarrea se vuelve grave o persistente, o si aprecia sangre en las heces, deje de usar Quinsair inmediatamente y consulte con su médico. No tome ningún medicamento contra la diarrea sin consultar antes con su médico.
- Resistencia a antibióticos
Las bacterias se pueden volver resistentes al tratamiento con un antibiótico con el tiempo. Esto significa que Quinsair no se debe usar para prevenir infecciones de pulmón. Solo se debe usar para tratar las infecciones de pulmón causadas por Pseudomonasaeruginosa.Consulte a su médico si tiene cualquier duda o preocupación al respecto.
- Sobreinfecciones
Algunas veces el tratamiento prolongado con antibióticos puede hacer que contraiga otra infección causada por otras bacterias que no se ven afectadas por el antibiótico (sobreinfección). Consulte a su médico si tiene cualquier duda o preocupación sobre la sobreinfección y el uso de Quinsair.
- Problemas de visión
Si nota cualquier cambio en la vista o cualquier otro problema en los ojos mientras esté usando Quinsair, póngase en contacto inmediatamente con un oftalmólogo.
- Fotosensibilidad
Quinsair puede hacer que su piel se vuelva más sensible a la luz solar. Debe evitar la exposición prolongada a la luz del sol y a la luz solar intensa, y no debe utilizar camas solares ni cualquier otra lámpara UV mientras esté usando Quinsair y durante 48 horas después de interrumpir el tratamiento.
- Resultados analíticos falsos
Determinadas pruebas (p. ej. para confirmar una tuberculosis o detectar analgésicos fuertes) pueden arrojar resultados erróneos mientras esté siendo tratado con Quinsair.
- Si le han diagnosticado un aumento de tamaño o un «bulto» de un vaso sanguíneo de gran tamaño (aneurisma aórtico o aneurisma de un vaso de gran tamaño periférico).
- Si ha sufrido un episodio previo de disección aórtica (desgarro de la pared de la aorta).
- Si se le ha diagnosticado una insuficiencia de la válvula cardíaca (regurgitación de las válvulas cardíacas).
- Si tiene antecedentes familiares de aneurisma aórtico o disección aórtica, enfermedad congénita de las válvulas cardíacas u otros factores de riesgo o condiciones predisponentes (p. ej., trastornos del tejido conjuntivo como el síndrome de Marfan o el síndrome vascular de Ehlers-Danlos, el síndrome de Turner o el síndrome de Sjögren (una enfermedad autoinmune inflamatoria), o trastornos vasculares como arteritis de Takayasu, arteritis de células gigantes, enfermedad de Behçet, hipertensión arterial o aterosclerosis conocida, artritis reumatoide (una enfermedad de las articulaciones) o endocarditis (una infección del corazón)).
- Si siente un dolor fuerte y repentino en el abdomen, el pecho o la espalda, que pueden ser síntomas de disección o aneurisma aórticos, acuda inmediatamente a un servicio de urgencias. Puede aumentar el riesgo si está recibiendo un tratamiento con corticosteroides sistémicos.
- Si empieza a experimentar una aparición repentina de disnea, especialmente cuando se tumba en la cama, o si observa hinchazón en los tobillos, los pies o el abdomen o la aparición de palpitaciones cardíacas (sensación de latido cardíaco rápido o irregular), debe informar a su médico inmediatamente.
Niños y adolescentes
Quinsair no se debe administrar a niños ni a adolescentes menores de 18 años, ya que no se dispone de suficiente información sobre su uso en este grupo de edad.
Otros medicamentos y Quinsair
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento. Estos medicamentos pueden interferir con los efectos de Quinsair.
Informe a su médicosi está tomando alguno de los siguientes medicamentos:
- Antagonistas de la vitamina K como la warfarina(usada para evitar la formación de coágulos en la sangre). Tomar estos medicamentos con Quinsair puede dar lugar a un aumento de las hemorragias. Su médico tal vez necesite realizarle análisis de sangre regulares para comprobar si su sangre coagula bien.
- Teofilina(usada para tratar problemas respiratorios) o antiinflamatorios no esteroideos (AINE) como fenbufeno, ácido acetilsalicílico(un principio activo presente en muchos medicamentos usados para aliviar el dolor y bajar la fiebre, así como para evitar que la sangre se coagule) o ibuprofeno. Tomar Quinsair al mismo tiempo que estos medicamentos puede incrementar el riesgo de que sufra un ataque (convulsión).
- Medicamentos como probenecid(usado para prevenir la gota) o cimetidina(usada para tratar las úlceras). Tomar Quinsair al mismo tiempo que estos medicamentos puede afectar a la manera en la que sus riñones procesan el medicamento, lo cual tiene especial importancia si padece problemas de riñón.
- Ciclosporina(usada después de los trasplantes de órganos) o medicamentos que afectan al ritmo cardiaco(como los antiarrítmicos, los antidepresivos tricíclicos, los antibióticos macrólidos y los antipsicóticos). Quinsair puede interferir con los efectos de estos medicamentos. Su médico se lo explicará con más detalle.
Embarazo y lactancia
Quinsair no se debe usar estando embarazada o en periodo de lactancia. Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Conducción y uso de máquinas
Quinsair le puede hacer sentirse mareado, cansado o débil, o causarle problemas de visión. Si le sucede esto, no conduzca ni use herramientas o máquinas.
3. Cómo usar Quinsair
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
¿Cúanto producto debo usar?
Inhale el contenido de una ampolla (240mg) dos veces al día utilizando el sistema nebulizador Zirela.Se tarda alrededor de 5 minutos en inhalar el medicamento con el nebulizador.
¿Cuándo debo usarlo?
Inhalar Quinsair a la misma hora cada día le ayudará a recordar cuándo tomar el medicamento. Inhale el medicamento como sigue:
- 1 ampolla por la mañana usando el nebulizador Zirela
- 1 ampolla por la noche usando el nebulizador Zirela
Lo ideal es dejar un intervalo de unas 12 horas entre las dosis.
¿Durante cuánto tiempo debo usarlo?
Debe usar Quinsair cada día durante 28 días y a continuación realizar un descanso de 28 días durante el cual no inhalará Quinsair. Después comenzará un nuevo ciclo de tratamiento.
Es importante que siga usando el medicamento dos veces al día durante los 28 días de tratamiento y que mantenga el ciclo de 28 días con tratamiento y 28 días sin tratamiento durante el tiempo que su médico le indique.


Si experimenta dificultades respiratorias al usar Quinsair ¿qué medicamento adicional le puede recetar su médico?
Si experimenta dificultades respiratorias después de usar Quinsair, su médico le puede recetar un inhalador que contenga un medicamento broncodilatador (p. ej. salbutamol). Inhale este medicamento al menos 15 minutos y hasta 4 horas antes de su siguiente dosis de Quinsair.
¿Qué ocurre si estoy usando varios inhaladores diferentes y otros tratamientos para la fibrosis quística?
Si está recibiendo varios tratamientos inhalados diferentes y otros tratamientos para la fibrosis quística, se recomienda que use los medicamentos en el orden siguiente:
1º Broncodilatadores
2º Dornasa alfa
3º Técnicas de permeabilización de la vía aérea
4º Quinsair
5º Esteroides inhalados
Cómo usarlo
Quinsair se debe administrar por inhalación utilizando un nebulizador de mano Zirela(que incluye un cabezal aerosol Zirela). Este debe estar conectado o bien a un controlador eBase o bien a una unidad de control eFlow rapid.
Información que es importante conocer antes de empezar
- Cada ampolla es solamente para un solo uso. Una vez abierta laampolla, el contenido se debe utilizar inmediatamente.
- No use Quinsair si nota que el sobre o las ampollas han sido manipulados.
- No use Quinsair si nota que está turbio o hay partículas en la disolución.
- No mezcle Quinsair con ningún otro medicamentoen el nebulizador de mano Zirela.
- No introduzca ningún otro medicamento distinto a Quinsair en el nebulizador de mano Zirela.
- No intente inhalar Quinsair utilizando ningún otro tipo de nebulizador de mano.
- Compruebe que su sistema nebulizador Zirela funciona correctamente antes de iniciar su tratamiento.
- No trague el líquido de la ampolla.
Lea cuidadosamente las instrucciones de uso del fabricante que acompañan a su nebulizador de mano Zirela.
¿Cómo debo preparar mi sistema nebulizador para inhalar el medicamento?
Guarde las instrucciones de uso de Zirela en lugar seguro, ya que detallan cómo montar el dispositivo.
- Asegúrese de colocar el nebulizador de mano Zirelasobre una superficie horizontal y estable.
- Introduzca todo el contenido de una ampollaen el depósito para el medicamento del nebulizador de mano Zirela (Figura 1). Asegúrese de vaciar la ampolla completamente, dando unos golpecitos contra el lado del depósito si fuera necesario.
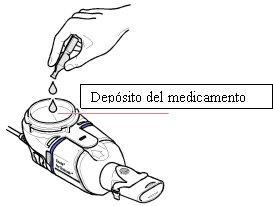
Figura1
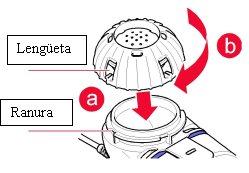 Cierre el depósito del medicamentoalineando las lengüetas de la tapa del medicamento con las ranuras del depósito (a). Oprima y gire la tapa en sentido horario hasta donde sea posible (b, Figura 2).
Cierre el depósito del medicamentoalineando las lengüetas de la tapa del medicamento con las ranuras del depósito (a). Oprima y gire la tapa en sentido horario hasta donde sea posible (b, Figura 2).
Figura2
¿Cómo debo usar el sistema nebulizador Zirela?
- Para comenzar su tratamiento,siéntese en una posición relajada y erguida.
- Sujete el nebulizador nivelado, pulse y mantenga pulsado el botón on/off del controlador durante unos pocos segundos. Oirá un pitido y la luz de estado se pondrá verde.
- Después de unos pocos segundos, una nube de aerosol empezará a fluirhasta la cámara del aerosol del nebulizador de mano Zirela. Si no empieza a fluir la nube de aerosol, intente solucionarlo consultando las instrucciones de uso del fabricante de Zirela.
- Manteniendo nivelado el nebulizador, introduzca la boquilla en la boca y cierre los labios alrededor de la misma (Figura 3).
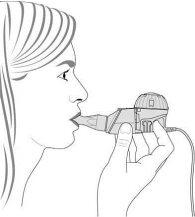
Figura3
- Respire normalmente(inhale y exhale) a través de la boquilla. Intente no respirar por la nariz. Continúe inhalando y exhalando cómodamente hasta que el tratamiento se haya acabado.Se tarda alrededor de 5 minutos en inhalar el medicamento con el nebulizador.
- Cuando se haya liberado todo el medicamento, oirá dos pitidos, que significan que el tratamiento se ha completado.
- Una vez completado, abra la tapa del medicamentopara asegurarse de que se ha utilizado todo el medicamento. Puede que queden algunas gotas de medicamento en el fondo del depósito al final del tratamiento. Esto es normal. No obstante, si queda más cantidad que unas gotas, vuelva a colocar la tapa y reanude el tratamiento.
- Una vez completado el tratamiento, desconecte el controlador y desmonte el nebulizador de mano Zirela para limpiarlo y desinfectarlo. Las instrucciones de uso del fabricante recogen todos los detalles sobre la limpieza y desinfección.
¿Qué ocurre si necesito parar el tratamiento antes de haber acabado?
Si por cualquier motivo necesita parar el tratamiento antes de que se acabe, pulse y mantenga pulsado el botón on/off del controlador durante un segundo. Una vez apagado del todo y cuando esté listo para reanudarlo, vuelva a pulsar y mantener pulsado el botón on/off durante un segundo. El tratamiento se reanudará. Ahora debe inhalar y exhalar a través de la boquilla como antes.
¿Cómo y cuándo debo sustituir en nebulizador de mano Zirela?
Se debe usar un nebulizador de mano Zirela durante un ciclo de tratamiento de 28 días. Consulte la información sobre limpieza y almacenamiento en las instrucciones de uso del fabricante.
Si usa más Quinsair del que debe
Si ha usado más Quinsair del que debe, informe de ello a su médico lo antes posible. Puede experimentar síntomas tales como latido cardiaco irregular, que su médico deberá comprobar. Si se traga el contenido de la ampolla, no se preocupe, pero informe a su médico lo antes posible.
Si olvidó usar Quinsair
Si se le olvida una dosis, adminístrela tan pronto como se acuerde, siempre y cuando quede un intervalo de 8 horas hasta la dosis siguiente. Si ya es casi la hora de la dosis siguiente, sáltese la dosis olvidada.
No inhale el contenido de más de una ampolla para compensar la dosis olvidada.
Si interrumpe el tratamiento con Quinsair
No deje de usar Quinsair sin consultar primero con su médico, ya que su infección pulmonar puede empeorar.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Algunos efectos adversos pueden ser graves
Si nota una reacción alérgica gravedespués de inhalar Quinsair, consiga de inmediato
atención médica urgente. Los síntomas incluyen:
- Comezón generalizado y sensación de calor que afecta especialmente al cuero cabelludo, la boca, la garganta, las palmas de las manos y las plantas de los pies.
- Sonidos silbantes importantes que se producen al respirar (sibilancias), o respiración ruidosa o dificultosa
- Urticaria o ronchas acusadas
- Hinchazón de los labios, la cara, la garganta o la lengua
- Color de la piel pálido o grisáceo
- Ritmo cardiaco acelerado
- Desmayo o desvanecimiento
Deje de usar Quinsair y póngase en contacto con su médico inmediatamente:
- si experimenta dolor, rigidez y/o hinchazón de las articulaciones
- si presenta problemas en el hígado. Los síntomas son:
- Pérdida de apetito
- Piel y ojos amarillentos (ictericia)
- Orina de color oscuro
- Comezón
- Dolor alrededor del estómago (abdomen)
Otras posibles reacciones adversas:
Muy frecuentes: pueden afectar a más de 1 de cada 10personas
- Tos
- Sentido del gusto anormal
- Cansancio, debilidad y menor tolerancia al ejercicio
- Pérdida de apetito y pérdida de peso
- Falta de aire al respirar
- Cambios en la cantidad y en la densidad del moco o la flema
- Tos con sangre
- Disminución de la cantidad de aire que se puede exhalar en un segundo (prueba VEF1 reducida)
Frecuentes: pueden afectar hasta 1 de cada 10personas
- Infección fúngica alrededor de la vagina
- Insomnio o dificultad para dormir
- Dolor de cabeza
- Mareo
- Zumbidos o ruidos en el oído (tinnitus)
- Cambios en la voz
- Náuseas y vómitos
- Dolor abdominal
- Diarrea
- Estreñimiento
- Exantema
- Dolor en las articulaciones (articular) o muscular
- Fiebre
- Resultados anormales de las pruebas sanguíneas (aumento de la concentración sanguínea de determinadas enzimas hepáticas o de la bilirrubina, y valores reducidos en la prueba de la función renal)
- Valores reducidos en la prueba de la función pulmonar
- Aumento o disminución de la cantidad de azúcar (glucosa) en sangre
- Ruidos respiratorios anormales
Poco frecuentes: pueden afectar hasta 1 de cada 100personas
- Infección por hongos en la boca
- Número reducido de glóbulos rojos en la sangre (anemia) o de las células en la sangre que la ayudan a coagular (plaquetas)
- Número reducido o aumentado de glóbulos blancos en la sangre
- Sentirse ansioso, inquieto o agitado y/o deprimido
- Reducción del sentido del olfato
- Somnolencia
- Cambios en la visión
- Pérdida de audición
- Aumento del ritmo cardiaco
- Dificultad para respirar
- Arcadas
- Indigestión
- Flatulencia
- Urticaria o ronchas y comezón
- Dolor de la pared del pecho (torácica)
- Insuficiencia renal
- Cambios en el ritmo cardiaco
- Dolor, quemazón, escozor, hormigueo, entumecimiento o debilidad en las extremidades (neuropatía)
Los siguientes efectos adversos también han sido comunicados después de tomar comprimidos o recibir una perfusión intravenosa que contenía levofloxacino, por lo que podrían aparecer después de usar Quinsair:
Poco frecuentes: pueden afectar hasta 1 de cada 100personas
- Sensación de confusión o nerviosismo
- Temblor
- Sensación de mareo, de dar vueltas o caerse (vértigo)
- Sudoración excesiva
Raros: pueden afectar hasta 1 de cada 1.000personas
- Alucinaciones y/o percepciones paranoides
- Sentirse agitado
- Sueños inusuales o pesadillas
- Convulsiones (ataques)
- Sensación de hormigueo (“agujas y alfileres”) y/o entumecimiento
- Palpitaciones
- Presión sanguínea baja
- Debilidad muscular
- Síndrome asociado con alteraciones en la eliminación de agua y niveles bajos de sodio
(SIADH)
- Erupción generalizada, temperatura corporal alta, elevación de las enzimas hepáticas,
anomalías sanguíneas (eosinofilia), ganglios linfáticos agrandados y otros órganos del
cuerpo implicados (reacción a fármaco con eosinofilia y síntomas sistémicos)
- Manchas eritematosas claramente delimitadas con o sin ampollas
Frecuencia desconocida: no puede estimarse a partir de los datos disponibles
- Número reducido de todos los tipos de células de la sangre
- Coma diabético
- Problemas mentales graves (en casos muy raros, pueden dar lugar a autolesiones)
- Dolor, quemazón, escozor, hormigueo, entumecimiento o debilidad en las extremidades (neuropatía)
- Movimientos musculares involuntarios, tics o espasmos
- Desvanecimiento
- Dolores de cabeza intensos punzantes con pérdida de la visión
- Pérdida temporal de la visión
- Latido de corazón rápido o anormal
- Inflamación de los pulmones
- Reacciones cutáneas graves como ampollas dolorosas o lesiones posiblemente en la boca, nariz o vagina
- Aumento de la sensibilidad de la piel a la luz solar o ultravioleta (camas solares u otras lámparas UV)
- Inflamación de los vasos sanguíneos
- Inflamación de la boca o los labios
- Degradación rápida de los músculos
- Inflamación de un tendón o rotura de un tendón
- Dolor, incluyendo dolor de espalda, en el pecho (torácico), de brazos y de piernas y brazos
La administración de antibióticos que contienen quinolonas y fluoroquinolonas se ha asociado a casos muy raros de reacciones adversas de larga duración (incluso meses o años) o permanentes, tales como inflamación de tendones, rotura de tendones, dolor en las articulaciones, dolor en las extremidades, dificultad para caminar, sensaciones anómalas, tales como pinchazos, hormigueo, cosquilleo, quemazón, escozor, entumecimiento o dolor (neuropatía), fatiga, disminución de la memoria y de la concentración, efectos sobre la salud mental (que pueden incluir trastornos del sueño, ansiedad, ataques de pánico, depresión e ideas de suicidio) y disminución de la audición, la vista, el gusto y el olfato, en algunos casos con independencia de la presencia de factores de riesgo preexistentes.
Se han notificado casos de aumento de tamaño y debilitamiento o desgarro de la pared aórtica (aneurismas y disecciones), lo que podría producir una rotura y llegar a ser mortal, e insuficiencia de válvulas cardíacas en pacientes que han recibido fluoroquinolonas. Ver también la sección 2.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano https://www.notificaRAM.eshttps://www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Quinsair
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la ampolla, el sobre de papel y las cajas después de CAD. La fecha de caducidad es el último día del mes que se indica.
Cada ampolla es solamente para un solo uso. Una vez abierta la ampolla, el contenido se debe utilizar inmediatamente. Cualquier producto no utilizado debe ser desechado. Vuelva a introducir en el sobre las ampollas sin usar y sin abrir para protegerlas de la luz.
Conservar en el envase original para protegerlo de la luz. Este medicamento no requiere ninguna temperatura especial de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Quinsair
- El principio activo es levofloxacino. Una ampolla contiene levofloxacino hemihidrato equivalente a 240 mg de levofloxacino.
- Los demás componentes son cloruro de magnesio hexahidrato y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Quinsair es una solución para inhalación por nebulizador transparente, de color amarillo pálido.
El medicamento viene preparado en pequeñas ampollas de plástico de 3 ml. Hay cuatro ampollas selladas en cada sobre de papel.
Quinsair se suministra en envases para 28 días (que contiene una caja interior con 56 (14 sobres de 4) ampollas) o en envases para 2 días (que contiene una caja interior con 8 (2 sobres de 4) ampollas) y una caja que contiene un nebulizador de mano Zirela con las instrucciones de uso del fabricante.
Puede que solamente estén comercializados algunos tamaños de envases.
La ampolla solo está etiquetada en inglés. La información que aparece en la ampolla es:
En la parte de delante del extremo de la ampolla
Quinsair 240 mg
Solución para inhalación por nebulizador
Levofloxacino
Vía inhalatoria 2,4 ml
En la “zona ondulada” a ambos lados del extremo de la ampolla
LOTE
CAD
Titular de la autorización de comercialización
Chiesi Farmaceutici S.p.A.
Via Palermo, 26/A
43122 Parma
Italia
Responsable de la fabricación
Adare Pharmaceuticals S.r.l.
Via Martin Luther King, 13
20060 Pessano con Bornago (MI)
Italia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Chiesi sa/nv Tél/Tel: + 32 (0)2 788 42 00 | Lietuva Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 |
| Luxembourg/Luxemburg Chiesi sa/nv Tél/Tel: + 32 (0)2 788 42 00 |
Ceská republika Chiesi CZ s.r.o. Tel: + 420 261221745 | Magyarország Chiesi Hungary Kft. Tel.: + 36-1-429 1060 |
Danmark Chiesi Pharma AB Tlf: + 46 8 753 35 20 | Malta Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 |
Deutschland Chiesi GmbH Tel: + 49 40 89724-0 | Nederland Chiesi Pharmaceuticals B.V. Tel: + 31 88 501 64 00 |
Eesti Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 | Norge Chiesi Pharma AB Tlf: + 46 8 753 35 20 |
Ελλ?δα Chiesi Hellas AEBE Τηλ: + 30 210 6179763 | Österreich Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 |
España Chiesi España, S.A.U. Tel: + 34 93 494 8000 | Polska Chiesi Poland Sp. z.o.o. Tel.: + 48 22 620 1421 |
France Chiesi S.A.S. Tél: + 33 1 47688899 | Portugal Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 |
Hrvatska Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 | România Chiesi Romania S.R.L. Tel: + 40 212023642 |
Ireland Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 | Slovenija Chiesi Slovenija d.o.o. Tel: + 386-1-43 00 901 |
Ísland Chiesi Pharma AB Sími: +46 8 753 35 20 | Slovenská republika Chiesi Slovakia s.r.o. Tel: + 421 259300060 |
Italia Chiesi Italia S.p.A. Tel: + 39 0521 279 | Suomi/Finland Chiesi Pharma AB Puh/Tel: +46 8 753 35 20 |
Κ?προς Chiesi Farmaceutici S.p.A. Τηλ: + 39 0521 2791 | Sverige Chiesi Pharma AB Tel: +46 8 753 35 20 |
Latvija Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 |
Fecha de la última revisión de este prospecto: Abril 2025.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu. También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a QUINSAIR 240 MG SOLUCION PARA INHALACION POR NEBULIZADORForma farmacéutica: COMPRIMIDO, 500 MGPrincipio activo: levofloxacinoFabricante: Especialidades Farmaceuticas Centrum S.A.Requiere recetaForma farmacéutica: COMPRIMIDO, 500 MGPrincipio activo: levofloxacinoFabricante: Mabo Farma S.A.Requiere recetaForma farmacéutica: COMPRIMIDO, 500 MGPrincipio activo: levofloxacinoFabricante: Accord Healthcare S.L.U.Requiere receta
Médicos online para QUINSAIR 240 MG SOLUCION PARA INHALACION POR NEBULIZADOR
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de QUINSAIR 240 MG SOLUCION PARA INHALACION POR NEBULIZADOR, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes
















