PROLIA 60 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar PROLIA 60 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Prolia 60mg solución inyectable en jeringa precargada
denosumab
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
- Su médico le proporcionará una tarjeta recordatorio para el paciente, que contiene información importante de seguridad que debe conocer antes y durante su tratamiento con Prolia.
Contenido del prospecto
- Qué es Prolia y para qué se utiliza
- Qué necesita saber antes de empezar a usar Prolia
- Cómo usar Prolia
- Posibles efectos adversos
- Conservación de Prolia
- Contenido del envase e información adicional
1. Qué es Prolia y cómo funciona y para qué se utiliza
Qué es Prolia y cómo funciona
Prolia contiene denosumab, una proteína (anticuerpo monoclonal) que interfiere en la acción de otra proteína con el objetivo de tratar la pérdida ósea y la osteoporosis. El tratamiento con Prolia refuerza los huesos y reduce las posibilidades de fractura.
El hueso es un tejido vivo que se renueva continuamente. Los estrógenos contribuyen a la conservación de la salud de los huesos. Después de la menopausia, el nivel de estrógenos desciende, lo que puede provocar que los huesos se vuelvan más finos y frágiles. A la larga esto puede provocar una enfermedad llamada osteoporosis. La osteoporosis también puede ocurrir en varones debido a varias causas incluyendo la edad y/o un nivel bajo de la hormona masculina, testosterona. También, se puede dar en pacientes en tratamiento con glucocorticoides. Muchos pacientes con osteoporosis no presentan síntomas, aunque siguen teniendo riesgo de fracturarse los huesos, sobre todo en la columna, la cadera y las muñecas.
Las intervenciones quirúrgicas o los medicamentos que detienen la producción de estrógeno o testosterona, utilizados para tratar pacientes con cáncer de próstata o de mama, también pueden provocar la pérdida ósea. Con ello, los huesos se hacen más débiles y se rompen con más facilidad.
Para qué se utiliza Prolia
Prolia se utiliza para tratar:
- la osteoporosis posterior a la menopausia (posmenopáusica) en mujeres y en varones que tienen un riesgo incrementado de fractura (rotura de huesos), reduciendo el riesgo de fracturas de la cadera, de la columna y en localizaciones que no son la columna.
- la pérdida ósea causada por la reducción del nivel hormonal (testosterona) como consecuencia de una operación quirúrgica o un tratamiento con medicamentos en pacientes con cáncer de próstata.
la pérdida ósea resultante del tratamiento a largo plazo con glucocorticoides en pacientes que tienen riesgo elevado de fractura.
2. Qué necesita saber antes de empezar a usar Prolia
No use Prolia:
- si tiene niveles bajos de calcio en la sangre (hipocalcemia).
- si es alérgico a denosumab o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Prolia.
Durante el tratamiento con Prolia usted podría desarrollar una infección de la piel con síntomas como una zona inflamada y enrojecida en la piel, más frecuentemente en la parte inferior de la pierna, que nota caliente y sensible al tacto (celulitis), y que puede ir acompañada de fiebre. Informe a su médico inmediatamente si presenta cualquiera de estos síntomas.
Además, debe tomar suplementos de calcio y vitamina D durante el tratamiento con Prolia. Su médico le comentará este aspecto.
Mientras está recibiendo Prolia podría presentar niveles bajos de calcio en la sangre. Informe a su médico inmediatamente si nota cualquiera de los siguientes síntomas: espasmos, contracciones o calambres musculares, y/o entumecimiento u hormigueo en los dedos de las manos, de los pies o alrededor de la boca, y/o convulsiones, confusión o pérdida de la conciencia.
En raras ocasiones, se han notificado casos de niveles muy bajos de calcio en sangre que han requerido hospitalización e, incluso, reacciones potencialmente mortales. Por lo tanto, antes de la administración de cada dosis y, en pacientes con predisposición a la hipocalcemia, en un plazo de dos semanas tras la dosis inicial, se comprobarán sus niveles de calcio en sangre (mediante un análisis de sangre).
Informe a su médico si tiene o ha tenido problemas renales graves, insuficiencia renal, si ha necesitado someterse a diálisis o si está tomando medicamentos llamados glucocorticoides (como prednisolona o dexametasona), ya que podrían incrementar el riesgo de tener niveles bajos de calcio en sangre si no toma suplementos de calcio.
Problemas en la boca, dientes o mandíbula
En pacientes que reciben Prolia para la osteoporosis se ha notificado en raras ocasiones (puede afectar hasta 1 de cada 1.000 personas) un efecto adverso llamado osteonecrosis mandibular (ONM) (daño en el hueso de la mandíbula). El riesgo de ONM aumenta en pacientes tratados durante mucho tiempo (puede afectar hasta 1 de cada 200 personas si son tratadas durante 10 años). La ONM también puede ocurrir después de interrumpir el tratamiento. Es importante intentar prevenir el desarrollo de la ONM ya que puede ser una afección dolorosa que puede ser difícil de tratar. Para reducir el riesgo de desarrollar ONM, siga estas precauciones:
Antes de recibir el tratamiento, informe a su médico o enfermero (profesional sanitario) si:
- tiene algún problema en su boca o dientes como mala salud dental, enfermedad de las encías, o una extracción dental planeada.
- no recibe revisiones dentales periódicas o hace tiempo que no se ha sometido a una revisión dental.
- es fumador (ya que puede incrementar el riesgo de problemas dentales).
- ha estado tratado previamente con un bisfosfonato (utilizado para prevenir o tratar trastornos óseos).
- está tomando medicamentos llamados corticosteroides (como prednisolona o dexametasona).
- tiene cáncer.
Su médico puede pedirle que se someta a una revisión dental antes de iniciar el tratamiento con Prolia.
Durante el tratamiento con Prolia, debe mantener una buena higiene bucal y someterse a revisiones dentales rutinarias. Si utiliza prótesis dental debe asegurarse de que esta se ajuste adecuadamente. Si está en tratamiento dental o se va a someter a cirugía dental (p. ej. extracciones dentales), informe a su médico sobre su tratamiento dental e informe a su dentista que está en tratamiento con Prolia.
Contacte con su médico y su dentista inmediatamente si experimenta cualquier problema en su boca o dientes como dientes móviles, dolor o inflamación, o úlceras que no curan o que supuran, ya que podrían ser síntomas de ONM.
Fracturas inusuales del fémur
Algunas personas han desarrollado fracturas inusuales en el fémur mientras estaban en tratamiento con Prolia. Consulte con su médico si sufre un dolor nuevo o inusual en la cadera, ingle o muslo.
Niños y adolescentes
Prolia no debería utilizarse en menores de 18 años de edad.
Uso de Prolia con otros medicamentos
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. Es especialmente importante que informe a su médico si está en tratamiento con otro medicamento que contenga denosumab.
No debe usar Prolia junto con otro medicamento que contenga denosumab.
Embarazo y lactancia
Prolia no se ha probado en mujeres embarazadas. Es importante que informe a su médico si está embarazada, cree que puede estarlo o planea quedarse embarazada. No se recomienda utilizar Prolia durante el embarazo. Las mujeres en edad fértil deben utilizar métodos anticonceptivos efectivos durante el tratamiento con Prolia y al menos 5 meses después de interrumpir el tratamiento con Prolia.
Si se queda embarazada durante el tratamiento con Prolia o menos de 5 meses después de interrumpir el tratamiento con Prolia, informe a su médico.
Se desconoce si Prolia se excreta en la leche materna. Es importante que le comunique a su médico si está en periodo de lactancia o si planea estarlo. Su médico le ayudará a decidir sobre si debe abandonar la lactancia materna, o si debe dejar de usar Prolia, teniendo en cuenta el beneficio de la lactancia materna para el niño y el beneficio de Prolia para la madre.
Si está en periodo de lactancia durante el tratamiento con Prolia, por favor informe a su médico.
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Conducción y uso de máquinas
La influencia de Prolia sobre la capacidad para conducir y utilizar máquinas es nula o insignificante.
Prolia contiene sorbitol
Este medicamento contiene 47 mg de sorbitol por cada ml de solución.
Prolia contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por 60 mg; esto es, esencialmente “exento de sodio”.
3. Cómo usar Prolia
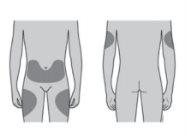
La dosis recomendada es una jeringa precargada de 60 mg administrada bajo la piel (vía subcutánea) en una inyección única una vez cada 6 meses. Los mejores lugares para ponerse la inyección son la parte superior de los muslos y el abdomen. Si la inyección se la pone un cuidador (persona que le atiende), también puede administrarle la inyección en la cara externa de la parte superior del brazo. Consulte con su médico la fecha de la siguiente posible inyección. Cada envase de Prolia contiene una tarjeta de recordatorio que puede despegarse del cartón y utilizarse para mantener un registro de la fecha de la siguiente inyección.
Además, debe tomar suplementos de calcio y vitamina D durante el tratamiento con Prolia. Su médico le comentará este aspecto.
Su médico podrá decidir si es mejor que la inyección de Prolia la administre usted o un cuidador. Su médico o profesional sanitario le mostrará a usted o a su cuidador cómo utilizar Prolia. Si desea obtener instrucciones sobre cómo inyectar Prolia, lea el último apartado de este prospecto.
No agitar.
Si olvidó usar Prolia
Si se salta una dosis de Prolia, la inyección deberá administrarse lo antes posible. Posteriormente, las inyecciones deberán programarse cada 6 meses a partir de la fecha de la última inyección.
Si interrumpe el tratamiento con Prolia
Para sacar el máximo beneficio de su tratamiento y reducir el riesgo de fracturas, es importante que utilice Prolia durante todo el periodo que le prescriba el médico. No interrumpa el tratamiento sin hablar antes con su médico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los pacientes tratados con Prolia pueden desarrollar infecciones en la piel (principalmente celulitis) con poca frecuencia. Informe a su médico inmediatamentesi aparece alguno de estos síntomas durante el tratamiento con Prolia: zona hinchada y enrojecida en la piel, normalmente en la parte inferior de la pierna, caliente y sensible al tacto y que puede ir acompañada de fiebre.
Raramente, los pacientes que reciben Prolia pueden desarrollar dolor en la boca y/o mandíbula, inflamación o úlceras que no se curan en la boca o mandíbula, supuración, entumecimiento o sensación de pesadez en la mandíbula, o movilidad de un diente. Estos podrían ser síntomas de daño óseo en la mandíbula (osteonecrosis). Informe a su médico y a su dentista inmediatamentesi experimenta tales síntomas mientras está en tratamiento con Prolia o después de interrumpir el tratamiento.
Raramente, los pacientes que reciben Prolia pueden presentar niveles bajos de calcio en sangre (hipocalcemia); los niveles muy bajos de calcio en sangre pueden requerir hospitalización e, incluso, podrían poner en peligro la vida. Los síntomas incluyen espasmos, contracciones o calambres en los músculos, y/o entumecimiento u hormigueo en los dedos de las manos, en los dedos de los pies o alrededor de la boca y/o convulsiones, confusión o pérdida de la conciencia. Si presenta alguno, informe a su médico inmediatamente. Los niveles bajos de calcio en la sangre también pueden provocar un cambio en el ritmo del corazón llamado prolongación del QT, que se puede observar realizando un electrocardiograma (ECG).
Raramente pueden darse fracturas inusuales del fémur en pacientes que reciben Prolia. Consulte con su médicosi sufre un dolor nuevo o inusual en la cadera, ingle o muslo ya que ello puede ser una indicación temprana de una posible fractura del fémur.
Raramente pueden darse reacciones alérgicas en pacientes que reciben Prolia. Los síntomas incluyen hinchazón en la cara, labios, lengua, garganta u otras partes del cuerpo; erupción, picor o urticaria en la piel, sibilancias o dificultad al respirar. Informe a su médicosi experimenta tales síntomas mientras está en tratamiento con Prolia.
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- dolor de huesos, articulaciones y/o músculos que a veces es intenso,
- dolor de piernas o brazos (dolor en las extremidades).
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas):
- micción dolorosa, micción frecuente, presencia de sangre en la orina, incontinencia urinaria,
- infección del tracto respiratorio superior,
- dolor, hormigueo o insensibilidad que se extiende hacia la parte inferior de la pierna (ciática),
- estreñimiento,
- molestias abdominales,
- erupción cutánea,
- afección cutánea con picor, enrojecimiento y/o sequedad (eccema),
- pérdida del pelo (alopecia).
Efectos adversos poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- fiebre, vómitos y dolor o molestias abdominales (diverticulitis),
- infección del oído,
- erupción en la piel o ulceraciones en la boca (erupciones liquenoides medicamentosas).
Efectos adversos muy raros(pueden afectar hasta 1 de cada 10.000 personas):
- reacción alérgica que puede dañar los vasos sanguíneos, principalmente de la piel (p. ej. manchas color púrpura o rojo parduzco, urticaria o úlceras de la piel) (vasculitis por hipersensibilidad).
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- consulte a su médico si usted tiene dolor de oído, el oído le supura y/o sufre una infección de oído. Estos podrían ser síntomas de daño en los huesos del oído.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Prolia
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en la caja después de “CAD” o “EXP”. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 ºC y 8 ºC).
No congelar.
Mantener la jeringa precargada en el embalaje exterior para protegerla de la luz.
Antes de la inyección, la jeringa precargada puede dejarse fuera de la nevera para que alcance la temperatura ambiente (hasta 25 ºC). De este modo la inyección será menos molesta. Una vez que la jeringa haya alcanzado la temperatura ambiente (hasta 25 ºC), debe utilizarse antes de que pasen 30 días.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Prolia
- El principio activo es denosumab. Cada jeringa precargada de 1 ml contiene 60 mg de denosumab (60 mg/ml).
- Los demás componentes son ácido acético glacial, hidróxido sódico, sorbitol (E420), polisorbato 20 y agua para preparaciones inyectables.
Aspecto de Prolia y contenido del envase
Prolia es una solución inyectable transparente, entre incolora y ligeramente amarilla, disponible en una jeringa precargada lista para su uso.
Cada envase contiene una jeringa precargada con protector de la aguja.
Cada envase contiene una jeringa precargada.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Amgen Europe B.V.
Minervum 7061,
4817 ZK Breda,
Países Bajos
Titular de la autorización de comercialización
Amgen Europe B.V.
Minervum 7061
4817 ZK Breda
Países Bajos
Fabricante
Amgen Technology (Ireland) Unlimited Company
Pottery Road
Dun Laoghaire
Co Dublin
Irlanda
Fabricante
Amgen NV
Telecomlaan 5-7
1831 Diegem
Bélgica
Puede solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien s.a. Amgen n.v. Tél/Tel: +32 (0)2 7752711 | Lietuva Amgen Switzerland AG Vilniaus filialas Tel: +370 5 219 7474 |
| Luxembourg/Luxemburg s.a. Amgen Belgique/Belgien Tél/Tel: +32 (0)2 7752711 |
Ceská republika Amgen s.r.o. Tel: +420 221 773 500 | Magyarország Amgen Kft. Tel.: +36 1 35 44 700 |
Danmark Amgen filial af Amgen AB, Sverige Tlf: +45 39617500 | Malta Amgen S.r.l. Italy Tel: +39 02 6241121 |
Deutschland Amgen GmbH Tel.: +49 89 1490960 | Nederland Amgen B.V. Tel: +31 (0)76 5732500 |
Eesti Amgen Switzerland AG Vilniaus filialas Tel: +372 586 09553 | Norge Amgen AB Tlf: +47 23308000 |
Ελλáδα Amgen Ελλ?ς Φαρμακευτικá Ε.Π.Ε. Τηλ: +30 210 3447000 | Österreich Amgen GmbH Tel: +43 (0)1 50 217 |
España Amgen S.A. Tel: +34 93 600 18 60 | Polska Amgen Biotechnologia Sp. z o.o. Tel.: +48 22 581 3000 |
France Amgen S.A.S. Tél: +33 (0)9 69 363 363 | Portugal Amgen Biofarmacêutica, Lda. Tel: +351 21 4220606 |
Hrvatska Amgen d.o.o. Tel: +385 (0)1 562 57 20 | România Amgen România SRL Tel: +4021 527 3000 |
Ireland Amgen Ireland Limited Tel: +353 1 8527400 | Slovenija AMGEN zdravila d.o.o. Tel: +386 (0)1 585 1767 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Amgen Slovakia s.r.o. Tel: +421 2 321 114 49 |
Italia Amgen S.r.l. Tel: +39 02 6241121 | Suomi/Finland Amgen AB, sivuliike Suomessa/Amgen AB, filial i Finland Puh/Tel: +358 (0)9 54900500 |
Kúπρος C.A Papaellinas Ltd Τηλ: +357 22741 741 | Sverige Amgen AB Tel: +46 (0)8 6951100 |
Latvija Amgen Switzerland AG Rigas filiale Tel: +371 257 25888 | United Kingdom (Northern Ireland) Amgen Limited Tel: +44 (0)1223 420305 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu/
-------------------------------------------------------------------------------------------------------------------
Instrucciones de uso: | |
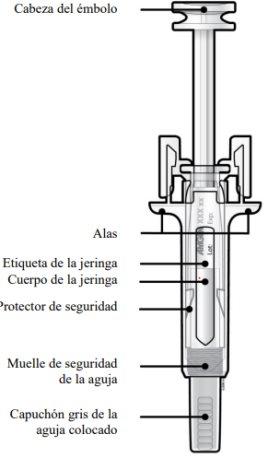
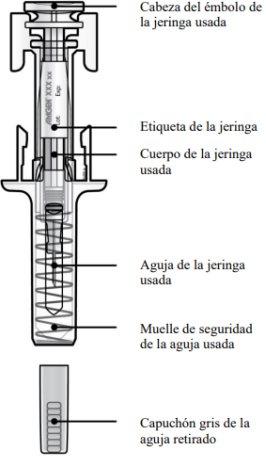
Guía de los componentes | |
Antes de usar | Después de usar |
|
|
Importante | |
Lea esta información importante antes de usar la jeringa precargada de Prolia con protector automático de la aguja:
Si tiene dudas contacte con su médico o profesional sanitario. | |
Paso 1: Preparación | |
A | Retire el envase de la jeringa precargada que hay en el interior del cartonaje y coja los materiales que necesite para su inyección: toallitas con alcohol, algodón o gasas, una tirita y un contenedor para desechar objetos punzantes (no incluido). |
Para una inyección menos molesta, deje la jeringa precargada a temperatura ambiente durante aproximadamente 30 minutos antes de la inyección. Lávese las manos cuidadosamente con agua y jabón. Coloque la jeringa precargada nueva y los otros materiales sobre una superficie limpia y bien iluminada.
|
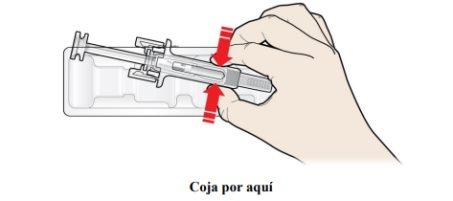
B | Abra el envase, retirando la cubierta. Coja la jeringa precargada por el protector de seguridad para sacarla del envase. |
| |
Por motivos de seguridad:
|
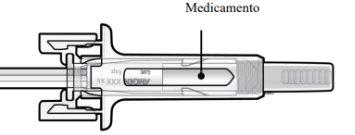
C | Examine el medicamento y la jeringa precargada. |
| |
Noutilice la jeringa precargada si:
| |
En cualquiera de estos casos, contacte con su médico o profesional sanitario. |
Paso 2: Prepárese | |
A | Lávese las manos cuidadosamente. Prepare y limpie el lugar de la inyección. |
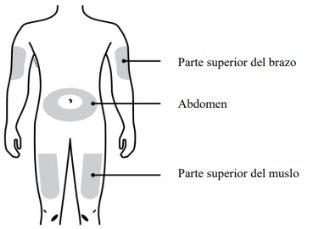
Puede inyectar el medicamento en:
| |
Limpie el lugar de la inyección con una gasa con alcohol. Deje que la piel se seque.
Evite inyectarse en áreas con cicatrices o estrías. | |
B | Tire cuidadosamente del capuchón gris de la aguja en línea recta manteniendo la jeringa separada de su cuerpo. |
|
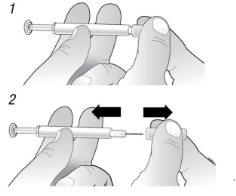
C | Pellizque el lugar de la inyección para crear una superficie firme. |
| |
Paso 3: Inyecte | |
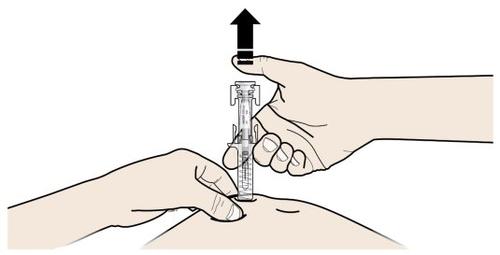
A | Mantenga la piel pellizcada. INSERTE la aguja en la piel. |
| |
B | PRESIONE la cabeza del émbolo con una presión ligera y constante hasta que sienta o escuche un “clic”. Empuje completamente hacia abajo hasta oír el “clic”. |
|
C | DEJE DE PRESIONAR la cabeza del émbolo. A continuación, SEPARE la jeringa de la piel. |
Tras soltar la cabeza del émbolo, el protector de seguridad de la jeringa precargada cubrirá de forma segura la aguja.
| |
Paso 4: Final | |
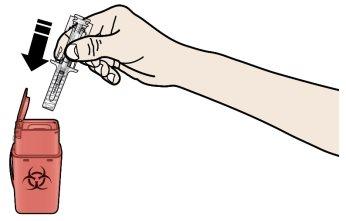
A | Deseche la jeringa precargada usada y otros materiales en un contenedor para desechar objetos punzantes. |
Los medicamentos deben ser eliminados de acuerdo con la normativa local. Pregunte a su farmacéutico cómo deshacerse de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente. Mantenga la jeringa y el contenedor de objetos punzantes fuera de la vista y del alcance de los niños.
|
B | Examine el lugar de la inyección. |
Si observa sangre, presione el lugar de la inyección con un algodón o una gasa. Nofrote el lugar de la inyección. Si es necesario, ponga una tirita. |
Instrucciones para inyectarse Prolia jeringa precargada
Esta sección contiene información sobre el modo de uso de la jeringa precargada de Prolia. Es importante que usted o su cuidador (persona que le atiende) no administren la inyección hasta que hayan recibido instrucciones sobre cómo hacerlo del médico o profesional sanitario.Lávese siempre las manos antes de poner la inyección. Si tiene dudas sobre cómo administrar la inyección, consulte a su médico o profesional sanitario.
Antes de empezar
Lea detenidamente todas las instrucciones antes de utilizar la jeringa precargada.
NOutilice la jeringa precargada si no tiene el capuchón de la aguja.
¿Cómo usar la jeringa precargada de Prolia?
Su médico le ha recetado una jeringa precargada de Prolia para inyectarla en el tejido que hay justo debajo de la piel (subcutáneo). Debe inyectarse todo el contenido (1 ml) de la jeringa precargada de Prolia una vez cada 6 meses, según las instrucciones de su médico.
Material:
Para administrar una inyección, necesitará:
- Una jeringa precargada nueva de Prolia;
- Algodón con alcohol o similar.
Qué debe hacer antes de administrar una inyección subcutánea de Prolia
- Saque la jeringa precargada de la nevera.
NOcoja la jeringa precargada por el émbolo o el capuchón de la aguja. Esto podría dañar el dispositivo.
- La jeringa precargada puede dejarse fuera de la nevera para que alcance la temperatura ambiente. De este modo la inyección será menos molesta.
NOla caliente de ningún otro modo, como por ejemplo en un microondas o en agua caliente.
NOdeje la jeringa expuesta a la luz solar directa.
- NOagite la jeringa precargada.
- NOquite el capuchón de la aguja de la jeringa precargada hasta que esté preparado para la inyección.
- Compruebe la fecha de caducidad en la etiqueta de la jeringa precargada (EXP).
NOla utilice si ya ha pasado el último día del mes indicado.
- Compruebe el aspecto de Prolia. Debe ser una solución transparente, entre incolora y ligeramente amarilla. No debe inyectarse si contiene partículas o si está turbia o descolorida.
- Busque una superficie limpia, cómoda y con buena iluminación y coloque todo el material necesario a su alcance.
- Lávese bien las manos.
¿Dónde debe administrarse la inyección? Los mejores lugares para inyectarse son la parte superior de los muslos y el abdomen. Su cuidador también puede administrarle la inyección en la cara externa de la parte superior de los brazos. |
|
¿Cómo se administra la inyección?
NOtoque la aguja ni presione el émbolo.
|
|
Recuerde:si tiene algún problema, no dude en pedir ayuda y consejo a su médico o profesional sanitario. Cómo deshacerse de las jeringas usadas
|
- País de registro
- Precio medio en farmacia208.66 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a PROLIA 60 mg SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 120 mgPrincipio activo: DenosumabFabricante: Fresenius Kabi Deutschland GmbhRequiere recetaForma farmacéutica: INYECTABLE, 120 mgPrincipio activo: DenosumabFabricante: Fresenius Kabi Deutschland GmbhRequiere recetaForma farmacéutica: INYECTABLE, 60 mgPrincipio activo: DenosumabFabricante: Fresenius Kabi Deutschland GmbhRequiere receta
Médicos online para PROLIA 60 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de PROLIA 60 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






 Nose inyecte en áreas donde la piel esté sensible, contusionada, enrojecida o con durezas.
Nose inyecte en áreas donde la piel esté sensible, contusionada, enrojecida o con durezas.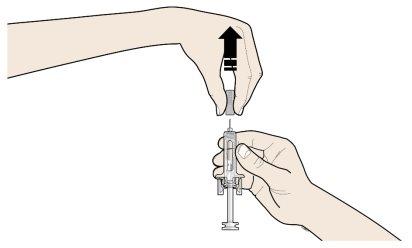
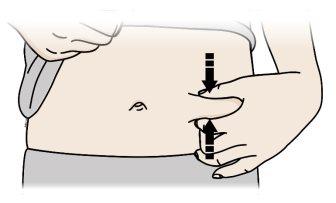
 Es importante mantener la piel pellizcada cuando se inyecte.
Es importante mantener la piel pellizcada cuando se inyecte.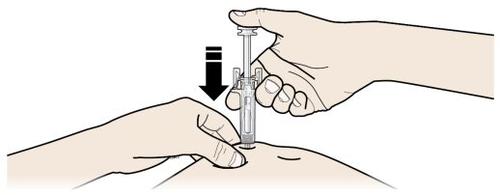
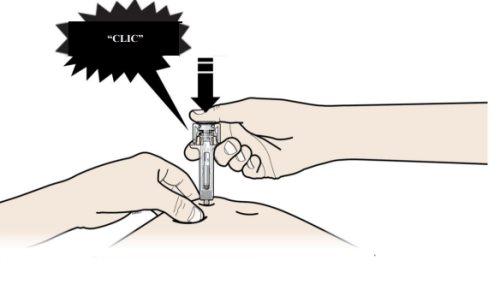
 Es importante presionar hacia abajo hasta oír el “clic” para recibir toda su dosis.
Es importante presionar hacia abajo hasta oír el “clic” para recibir toda su dosis.