
PROCTOSTEROID 10 MG ESPUMA RECTAL

Cómo usar PROCTOSTEROID 10 MG ESPUMA RECTAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Proctosteroid 10 mg espuma rectal
Triamcinolona diacetato
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- 1. Qué es Proctosteroid y para qué se utiliza
- 2. Qué necesita saber antes de empezar a usar Proctosteroid
- 3. Cómo usar Proctosteroid
- 4. Posibles efectos adversos
- 5. Conservación de Proctosteroid
- 6. Contenido del envase e información adicional
1. Qué es Proctosteroid y para qué se utiliza
Proctosteroid pertenece a un grupo de medicamentos denominados antiinflamatorios intestinales que desarrolla su acción antiinflamatoria sobre la mucosa rectal. Este medicamento está indicado en adultos para:
Tratamiento de colitis ulcerosa, proctosigmoiditis y proctitis granular.
Tratamiento de procesos inflamatorios rectales.
2. Qué necesita saber antes de empezar a usar Proctosteroid
No use Proctosteroid:
- Si es usted alérgico a la triamcinolona diacetato o a cualquier de los demás componentes del medicamento.
- Si tiene tuberculosis, varicela, herpes simple agudo.
- Si tiene abscesos, fístulas extensas, suturas intestinales recientes.
- Si tiene obstrucción intestinal.
- Si tiene peritonitis.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Proctosteroid.
- Si ha dejado el tratamiento con corticosteroides sistemáticos, al instaurar el tratamiento con Proctosteroid pueden producirse alteraciones funcionales del eje hipotalámico-hipofisario-suprarrenal.
- Póngase en contacto con su médico si presenta visión borrosa u otras alteraciones visuales.
Uso de Proctosteroid con otros medicamentos
Informe a su médico si está tomando o ha tomado recientemente otros medicamentos, incluso los adquiridos sin receta.
Algunos medicamentos pueden aumentar los efectos de Proctosteroid, por lo que su médico le hará controles minuciosos si está tomando estos medicamentos (incluidos algunos para el VIH: ritonavir, cobicistat).
Uso en deportistas:
Se informa a los deportistas que este medicamento contiene triamcinolona diacetato que puede producir un resultado positivo en las pruebas de control de dopaje.
Embarazo y lactancia:
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No existe evidencia de que Proctosteroid pueda perjudicar a la madre o al niño cuando se utiliza en el embarazo o en el periodo de lactancia. No obstante, debe contactar con su médico lo ante posible si se queda embarazada durante el tratamiento con Proctosteroid.
Conducción y uso de máquinas
Proctosteroid no afecta a su capacidad de conducir ni de utilizar herramientas o máquinas.
Información importante sobre algunos de los componentes de Proctosteroid:
Este medicamento contiene 210 mg de propilenglicol en cada aplicación. El propilenglicol puede provocar irritación de la piel.
Puede producir reacciones alérgicas (positivamente retardadas) porque contiene parahidroxibenzoato de metilo (E-218).
3. Cómo usar Proctosteroid
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico. En caso de duda, consulte a su médico o farmacéutico.
La dosis recomendada es:
Una aplicación dentro del recto una o dos veces al día, preferentemente después de una evacuación.
Intercálense pausas de varios días sin tratamiento cada dos o tres semanas.
Una vez obtenida la remisión de los síntomas, suele ser suficiente una aplicación en días alternos.
Instrucciones de administración
Leer cuidadosamente las instrucciones antes de utilizar por primera vez Proctosteroid.
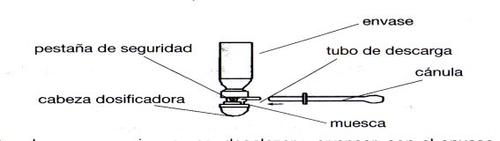
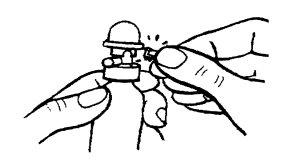
Antes de utilizar por primera vez, desplazar y arrancar, con el envase en posición vertical, la pestaña de seguridad de la cabeza dosificadora.
- A partir de este momento y siempre que se vaya a usar colocar el envase en posición vertical, tal y como indican los siguientes dibujos.
Conectar firmemente la cánula al tubo de descarga del aerosol y alinear la muesca situada debajo de la cabeza dosificadora del tubo de descarga.
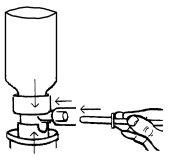
- Agitar enérgicamente el envase antes de usar.
- Sostener el envase en la palma de la mano con los dedos índice y corazón sobre la cabeza dosificadora (el producto sólo se puede dispensar cuando el aerosol está invertido con la cabeza hacia abajo y la mueca alineada).
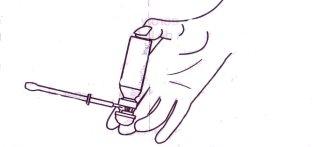
- Insertar el aplicador en el recto (puede lubricarse el extremo del aplicador para mayor comodidad). Una forma de utilizar el aerosol es elevando un pie sobre un taburete o silla, antes de introducir la cánula.
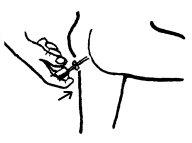
- Para administrar una dosis, presionar completamente la cabeza dosificadora una vez y liberarla. Esperar al menos 15 segundos antes de retirar el aplicador. Separa la cánula y deshecharla..
Si usted usa más Proctosteroid del que debe:
Si usted usa más Proctosteroid del que debe, consulte inmediatamente a su médico o farmacéutico.
El uso excesivo o prolongado de corticosteroides tópicos y de espuma endorrectales puede ocasionar efectos generales sistémicos por absorción del corticosteroide, como por ejemplo, el síndrome de Cushing, supresión suprarrenal, retraso en el crecimeinto de niños y adolescentes, disminución de la densidad mineral ósea, cataratas, glaucoma, entre otros.
La interrupción de la aplicación del fármaco corrige rápidamente los efectos cortisónicos.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usarProctosteroid:
No use una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos son, en general, leves y transitorios.
Efectos adversos poco frecuentes (pueden afectar hasta 1 de cada 100 personas)
- irritación en la zona de administración
- prurito en la zona de administración
- sindrome de Cushing
- hiperglucemia
- osteoporosis
- trastorno del colágeno (pérdida de colágeno).
Efecto adverso con una frecuencia no conocida (no puede estimarse la frecuencia a partir de los datos disponibles)
- visión borrosa.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano. https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Proctosteroid
Mantener este medicamento fuera de la vista y el alcance de los niños.
No conservar a temperatura superior a 30ºC.
No congelar o refrigerar.
Envase a presión.
Debe protegerse de la luz solar directa y no debe destruirse o quemarse, ni siquiera cuando esté vacío.
No utilice Proctosteroid después de la fecha de caducidad que aparece en el envase después de CAD.: La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punta SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Proctosteroid 10 mg espuma rectal
El principio activo es triamcinolona diacetato
Cada aplicación contiene aproximadamente una dosis de 10 mg de triamcinolona diacetato.
Los demás componentes son:
Cera emulsionante, macrogolglicéridos de caprilocarproilo, polisorbato 20, propilenglicol,parahidroxibenzoato de metilo (E-218), fosfato disódico, ácido cítrico anhidro, agua purificada y 1,1,1,2-tetrafluoretano.
Aspecto del producto y contenido del envase:
Proctosteroid es una espuma blanca o prácticamente blanca.
Se presenta en envase de 20 g y 16 cánulas aplicadoras.
Titular de la autorización de comercialización y responsable de la fabricación:
Laboratorio Aldo-Unión, S.L.
Calle Baronesa de Maldá, 73
08950 Esplugues de Llobregat
BARCELONA – ESPAÑA
Fecha de la última revisión de este prospecto: 02/2019
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
- País de registro
- Precio medio en farmacia10.93 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a PROCTOSTEROID 10 MG ESPUMA RECTALForma farmacéutica: COMPRIMIDO LIBERACION MODIFICADA, 5 mg/comprimidoPrincipio activo: beclometasoneFabricante: Promedica S.R.L.Requiere recetaForma farmacéutica: COMPRIMIDO LIBERACION MODIFICADA, 5 mg/comprimidoPrincipio activo: beclometasoneFabricante: Chiesi España S.A.U.Requiere recetaForma farmacéutica: LIQUIDO RECTAL, 2 mg budesonidaPrincipio activo: BudesonidaFabricante: Tillotts Pharma GmbhRequiere receta
Médicos online para PROCTOSTEROID 10 MG ESPUMA RECTAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de PROCTOSTEROID 10 MG ESPUMA RECTAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















