
ПРИОРИКС, ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ

Спросите врача о рецепте на ПРИОРИКС, ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ

Инструкция по применению ПРИОРИКС, ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ
Введение
Инструкция: информация для пользователя
Приорикс, порошок и растворитель в предварительно заполненной шприце для инъекционного раствора
Вакцина против кори, эпидемического паротита и краснухи (живая)
Прочитайте внимательно всю инструкцию перед тем, как вы получите эту вакцину, поскольку она содержит важную информацию для вас.
- Сохраните эту инструкцию, поскольку вам может потребоваться прочитать ее снова.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом или фармацевтом.
- Эта вакцина была назначена только вам, и вы не должны давать ее другим людям.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом или фармацевтом, даже если это побочные эффекты, которые не указаны в этой инструкции. См. раздел 4.
Эта инструкция была написана с учетом того, что человек, который ее читает, является тем, кто получает вакцину, но она может быть введена взрослым и детям, поэтому вы можете читать ее для своего ребенка.
Содержание инструкции
- Что такое Приорикс и для чего он используется
- Что вам нужно знать перед тем, как вы получите Приорикс
- Как вводится Приорикс
- Возможные побочные эффекты
- Хранение Приорикса
Содержание упаковки и дополнительная информация
1. Что такое Приорикс и для чего он используется
Приорикс - это вакцина, которая вводится детям от 9 месяцев, подросткам и взрослым для защиты от заболеваний, вызванных вирусами кори, эпидемического паротита и краснухи.
Как работает Приорикс
Когда человек вакцинируется Приориксом, его иммунная система (естественная система защиты организма) произведет антитела для защиты человека от инфекции вирусами кори, эпидемического паротита и краснухи.
Хотя Приорикс содержит живые вирусы, они слишком ослаблены, чтобы вызвать корь, эпидемический паротит или краснуху у здоровых людей.
2. Что вам нужно знать перед тем, как вы получите Приорикс
Приорикс не должен вводиться, если
- вы аллергичны к активным веществам или любому другому компоненту этой вакцины (указанным в разделе 6). Признаки аллергической реакции могут включать кожную сыпь с зудом, трудности с дыханием и отек лица или языка;
- вы аллергичны к неомицину (антибиотику). Хотя известная контактная дерматит (кожная сыпь, возникающая при прямом контакте кожи с аллергенами, такими как неомицин) не должна быть проблемой, сначала проконсультируйтесь с вашим врачом;
- у вас есть тяжелая инфекция с высокой температурой. В этих случаях вакцинация будет отложена до вашего выздоровления. Хотя легкая инфекция, такая как простуда, не должна быть проблемой, сначала проконсультируйтесь с вашим врачом;
- у вас есть какое-либо заболевание (например, ВИЧ или СИДА) или вы принимаете лекарство, которое может ослабить иммунную систему. То, что вы получите вакцину, будет зависеть от уровня ваших защитных сил;
- вы беременны. Кроме того, следует избегать беременности в течение 1 месяца после вакцинации.
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом или фармацевтом перед тем, как вы будете вакцинированы Приориксом, если:
- у вас есть нарушения центральной нервной системы, история судорог с высокой температурой или семейная история судорог. В случае высокой температуры после вакцинации немедленно проконсультируйтесь с вашим врачом;
- вы когда-либо имели тяжелую аллергическую реакцию на белки яйца;
- вы когда-либо имели побочный эффект после вакцинации против кори, эпидемического паротита или краснухи, который привел к появлению синяков или кровотечения в течение более длительного периода, чем обычно (см. раздел 4);
- у вас ослабленная иммунная система (например, ВИЧ-инфекция). Вас должны внимательно наблюдать, поскольку реакция на вакцину может быть недостаточной для обеспечения защиты от заболевания (см. раздел 2 «Приорикс не должен вводиться, если»).
До или после любой инъекции может произойти обморок (особенно у подростков), поэтому вы должны сообщить вашему врачу или медсестре, если вы когда-либо имели обморок после введения инъекции.
Если вы вакцинированы в течение 72 часов после контакта с человеком, больным корью, Приорикс защитит вас от заболевания до определенной степени.
Дети младше 12 месяцев
Возможно, что дети, вакцинированные в течение первого года жизни, не будут полностью защищены. Ваш врач посоветует, необходимы ли дополнительные дозы вакцины.
Как и все вакцины, возможно, что Приорикс не полностью защитит всех вакцинированных людей.
Использование Приорикса с другими лекарствами
Сообщите вашему врачу или фармацевту, если вы используете, недавно использовали или можете использовать любое другое лекарство (или другие вакцины).
Приорикс может быть введен вам одновременно с другими вакцинами, такими как дифтерия, тетанус, коклюш, Haemophilus influenzaeтип b, полiomielит, гепатит А, гепатит В, ветрянка, менингококковые вакцины группы Б, а также менингококковые вакцины группы С, менингококковые вакцины групп А, С, W-135, Y и конъюгированная пневмококковая вакцина. Проконсультируйтесь с вашим врачом или медсестрой для получения более подробной информации.
Должно быть использовано отдельное место для инъекции каждой вакцины.
Если они не вводятся одновременно, рекомендуется интервал не менее 1 месяца между введением Приорикса и другими живыми аттенуированными вакцинами.
Ваш врач может отложить вакцинацию не менее чем на 3 месяца, если вы получили переливание крови или человеческие антитела (иммуноглобулины).
Если необходимо провести тест на туберкулин, его следует провести до, одновременно с введением вакцины или через 6 недель после вакцинации Приориксом.
Беременность, лактация и фертильность
Приорикс не должен вводиться беременным женщинам.
Если вы беременны или кормите грудью, считаете, что можете быть беременной или планируете стать беременной, проконсультируйтесь с вашим врачом или фармацевтом перед тем, как вам будет введена вакцина. Также важно, чтобы вы не стали беременной в течение 1 месяца после вакцинации. В течение этого времени следует использовать эффективный метод контрацепции, чтобы избежать беременности.
В случае непреднамеренной вакцинации беременных женщин Приориксом, это не должно быть причиной для прерывания беременности.
Приорикс содержит сорбитол, пара-аминобензойную кислоту, фенилаланин, натрий и калий
Эта вакцина содержит 9 мг сорбитола на дозу.
Приорикс содержит пара-аминобензойную кислоту. Она может вызывать аллергические реакции (возможно, задержанные) и, в исключительных случаях, бронхоспазм.
Эта вакцина содержит 334 микрограмма фенилаланина на дозу. Фенилаланин может быть вредным в случае фенилкетонурии (ФКН), редкого генетического заболевания, при котором фенилаланин накапливается из-за того, что организм не может правильно удалить его.
Эта вакцина содержит менее 23 мг натрия (1 ммоль) на дозу; это означает, что она практически не содержит натрия.
Эта вакцина содержит менее 39 мг (1 ммоль) калия на дозу, поэтому она считается практически не содержащей калия.
3. Как вводится Приорикс
Приорикс вводится под кожу или в мышцу, либо в верхнюю часть руки, либо в наружную часть бедра.
Приорикс предназначен для детей от 9 месяцев, подростков и взрослых.
Ваш врач определит время и количество инъекций, подходящих для вас, на основе официальных рекомендаций.
Вакцина никогда не должна вводиться в вену.
4. Возможные побочные эффекты
Как и все лекарства, эта вакцина может вызывать побочные эффекты, хотя не все люди испытывают их.
Побочные эффекты, которые произошли в клинических испытаниях с Приориксом, были следующими:
- Очень частые (могут возникать более чем в 1 случае из 10 доз вакцины):
- покраснение в месте инъекции
- лихорадка 38°C или выше.
- Частые (могут возникать до 1 случая из 10 доз вакцины):
- боль и отек в месте инъекции
- лихорадка выше 39,5°C
- кожная сыпь (пятна)
- инфекция верхних дыхательных путей.
- Редкие (могут возникать до 1 случая из 100 доз вакцины):
- инфекция среднего уха
- отек лимфатических узлов (узлы в шее, подмышке или паху)
- потеря аппетита
- нервозность
- необычный плач
- бессонница
- покраснение, раздражение и влажность глаз (конъюнктивит)
- бронхит
- кашель
- отек околоушных желез (желез в щеках)
- диарея
- рвота.
- Очень редкие (могут возникать до 1 случая из 1 000 доз вакцины):
- судороги с высокой температурой
- аллергические реакции.
После выпуска Приорикса на рынок в некоторых случаях были зарегистрированы следующие побочные эффекты:
- боль в суставах и мышцах
- появление небольших кровяных пятен или синяков на коже с большей легкостью, чем обычно, из-за снижения количества тромбоцитов
- внезапная аллергическая реакция с риском для жизни
- инфекция или воспаление мозга, спинного мозга и периферических нервов, вызывающие временную трудность при ходьбе (неустойчивость) и/или временную потерю контроля над движениями тела, воспаление некоторых нервов, возможно с онемением или потерей чувствительности или движения (синдром Гийена-Барре)
- сужение или блокировка кровеносных сосудов
- эритема мультиформе (симптомы - красные пятна, часто сопровождаемые зудом, похожие на кожную сыпь, вызванную корью, начинающиеся на конечностях и иногда на лице и остальной части тела)
- симптомы, похожие на корь и эпидемический паротит (включая болезненный и временный отек яичек и отек желез в шее)
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, проконсультируйтесь с вашим врачом или фармацевтом, даже если это возможные побочные эффекты, которые не указаны в этой инструкции. Вы также можете сообщить о них напрямую через систему фармаковигиланса для лекарственных средств для человека, www.notificaRAM.es. Сообщая о побочных эффектах, вы можете внести свой вклад в предоставление более подробной информации о безопасности этого лекарства.
5. Хранение Приорикса
Храните эту вакцину вне поля зрения и досягаемости детей.
Не используйте эту вакцину после даты истечения срока годности, указанной на упаковке, после «Срок годности».
Храните и перевозите при охлаждении (между 2°C и 8°C).
Не замораживайте.
Храните в оригинальной упаковке, чтобы защитить от света.
Вакцина должна быть введена сразу после восстановления. Если это невозможно, ее следует хранить в холодильнике (между 2°C и 8°C) и использовать в течение 8 часов после восстановления.
Лекарства не должны выбрасываться в канализацию или мусор. Поместите упаковку и лекарства, которые вам больше не нужны, в пункт сбора SIGRE в аптеке. Если у вас есть сомнения, спросите вашего фармацевта, как избавиться от упаковки и лекарств, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
Состав Приорикса
- Активные вещества: живые аттенуированные вирусы кори, эпидемического паротита и краснухи.
- Другие компоненты:
Порошок: аминокислоты (содержат фенилаланин), лактоза (безводная), маннитол (Е-421), сорбитол (Е-420), среда 199 (содержит фенилаланин, пара-аминобензойную кислоту, натрий и калий).
Растворитель: вода для инъекций.
Внешний вид продукта и содержание упаковки
Приорикс выпускается в виде порошка и растворителя для инъекционного раствора (порошок в одном флаконе для 1 дозы и растворитель в предварительно заполненной шприце (0,5 мл)), с или без игл, в следующих размерах упаковки:
- с 2 отдельными иглами: упаковки по 1 или 10
- без иглы: упаковки по 1 или 10.
Приорикс поставляется в виде белого или слегка розового порошка, часть которого может быть желтоватой или слегка оранжевой, и прозрачной и бесцветной воды для инъекций для восстановления вакцины.
Возможно, что будут продаваться только некоторые размеры упаковки.
Владелец разрешения на маркетинг и производитель
Владелец разрешения на маркетинг:
GlaxoSmithKline, S.A.
PTM - C/ Severo Ochoa, 2
28760 Tres Cantos, Madrid
Телефон: 900 202 700
Факс: 91 807 03 10
электронная почта: [email protected]
Производитель:
GlaxoSmithKline Biologicals S.A.
Rue de l'Institut 89; 1330 Rixensart
Бельгия
Дата последнего обновления этой инструкции:07/2025
Подробная и актуальная информация о этом лекарстве доступна на сайте Агентства по лекарственным средствам и медицинским изделиям Испании (AEMPS) http://www.aemps.gob.es/
-----------------------------------------------------------------------------------------------------------------------------
Эта информация предназначена только для медицинских специалистов:
Как и все инъекционные вакцины, следует всегда иметь под рукой медицинское лечение и наблюдение, чтобы предотвратить возникновение редкой анафилактической реакции после введения вакцины.
Поскольку алкоголь и другие дезинфицирующие средства могут инактивировать живые аттенуированные вирусы вакцины, перед введением вакцины они должны быть удалены с кожи.
Приорикс не должен вводиться внутривенно ни при каких обстоятельствах.
В отсутствие данных о совместимости это лекарство не должно смешиваться с другими.
Перед восстановлением или введением следует визуально осмотреть растворитель и восстановленную вакцину, чтобы обнаружить любые посторонние частицы и/или изменение физического состояния. Если обнаружены какие-либо из них, не используйте растворитель или восстановленную вакцину.
Вакцина должна быть восстановлена путем добавления всего содержимого предварительно заполненной шприцы к флакону с порошком.
Чтобы узнать, как вставить иглу в шприц, внимательно прочитайте инструкции, предоставленные с изображениями 1 и 2. Однако шприц, поставляемый с Приориксом, может быть немного khác (без резьбы для винта). В этом случае игла должна быть вставлена без винта.
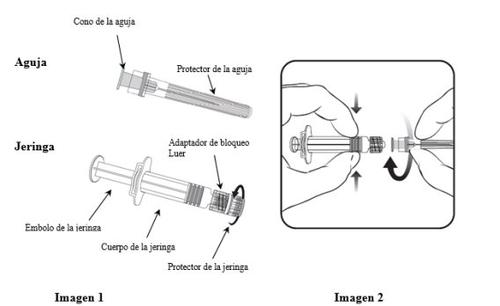
Всегда держите шприц за корпус, а не за поршень или адаптер Luer (ABL), и держите иглу на оси шприца (как показано на изображении 2). В противном случае ABL может быть деформирован и вызвать утечку.
Если во время сборки шприца ABL отсоединяется, используйте новую дозу вакцины (новый шприц и флакон).
- Поворачивайте защитный колпачок шприца против часовой стрелки (как показано на изображении 1).
Независимо от того, поворачивается ли ABL или нет, следуйте следующим шагам:
- Вставьте иглу в шприц, осторожно вставив конус иглы в ABL, и поверните на четверть оборота по часовой стрелке, пока не почувствуете, что она заблокирована (как показано на изображении 2).
- Удалите защитный колпачок иглы (может быть трудно).
- Добавьте растворитель к порошку. Хорошо встряхните смесь, пока порошок не будет полностью растворен.
Из-за незначительных вариаций pH вакцины восстановленная вакцина может иметь цвет от светло-оранжевого до розового фуксии, что не влияет на эффективность вакцины.
- Удалите все содержимое флакона.
- Для введения вакцины следует использовать новую иглу. Поворачивайте иглу в шприце и вставьте иглу для инъекции, повторив шаг 2 выше.
Вакцина должна быть введена сразу после восстановления. Если это невозможно, ее следует хранить при охлаждении (между 2°C и 8°C) и использовать в течение 8 часов после восстановления.
Утилизация непотребованного лекарства и всех материалов, которые были в контакте с ним, должна осуществляться в соответствии с местными правилами.
- Страна регистрации
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги ПРИОРИКС, ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА В ПРЕДНАПОЛНЕННОМ ШПРИЦЕАктивное вещество: measles, combinations with mumps and rubella, live attenuatedПроизводитель: Merck Sharp & Dohme B.V.Требуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 1 MILES DICT50 ВИРУС / 0,01 MILES DICT50 ВИРУС / 12,5 MILES DICT50 ВИРУСАктивное вещество: measles, combinations with mumps and rubella, live attenuatedПроизводитель: Merck Sharp & Dohme B.V.Требуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 3 Инфекционная доза 50% в культуре тканей / 3 Инфекционная доза 50% в культуре тканей / 4,3 Инфекционная доза 50% в культуре тканей / 3,99 ЕД ФИПАктивное вещество: measles, combinations with mumps, rubella and varicella, live attenuatedПроизводитель: Merck Sharp & Dohme B.V.Требуется рецепт
Аналоги ПРИОРИКС, ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог ПРИОРИКС, ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ в Польша
Аналог ПРИОРИКС, ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ в Украина
Врачи онлайн по ПРИОРИКС, ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на ПРИОРИКС, ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ – по решению врача и с учетом местных правил.














