
PEGASYS, 180 microgramos, SOLUCION INYECTABLE EN JERINGA PRECARGADA


Cómo usar PEGASYS, 180 microgramos, SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto:información para el usuario
Pegasys 90microgramos solución inyectable en jeringa precargada
Pegasys 135microgramos solución inyectable en jeringa precargada
Pegasys 180microgramos solución inyectable en jeringa precargada
peginterferón alfa-2a
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento,porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Pegasys y para qué se utiliza
- Qué necesita saber antes de empezar a usar Pegasys
- Cómo usar Pegasys
- Posibles efectos adversos
- Conservación de Pegasys
- Contenido del envase e información adicional
1. Qué es Pegasys y para qué se utiliza
Pegasys contiene el principio activo peginterferón alfa-2a, el cual es un interferón de larga duración de acción. El interferón es una proteína que modifica la respuesta del sistema inmune para ayudar a combatir infecciones y enfermedades graves y para impedir el crecimiento de las células cancerosas.
Pegasys se utiliza para tratar la policitemia vera y la trombocitemia esencial en adultos.
La policitemia vera y la trombocitemia esencial son tipos raros de cáncer en los que la médula ósea produce demasiados glóbulos rojos, glóbulos blancos y plaquetas (células que facilitan la coagulación de la sangre).
Policitemia vera y trombocitemia esencial:Pegasys se usa solo.
Pegasys también se emplea para tratar la hepatitis B o la hepatitis C crónica en adultos. También se utiliza para tratar la hepatitis B crónica en niños y adolescentes a partir de 3 años y la hepatitis C crónica en niños y adolescentes a partir de 5 años, que no han sido tratados antes. Tanto la hepatitis B como la C son infecciones virales que afectan al hígado.
Hepatitis B crónica:Pegasys se suele utilizar solo.
Hepatitis C crónica:Pegasys se usa en combinación con otros medicamentos, para el tratamiento de la hepatitis C crónica (HCC).
Debe consultar también el prospecto de los medicamentos que se usan en combinación con Pegasys.
2. Qué necesita saber antes de empezar a usar Pegasys
No use Pegasys
- si es alérgico al peginterferón alfa-2a, a cualquier interferón o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si ha tenido alguna vez un ataque al corazón o ha sido hospitalizado por dolores de pecho graves en los últimos seis meses.
- si tiene o ha tenido alguna vez una enfermedad autoinmunitaria (como artritis reumatoide, psoriasis o enfermedad inflamatoria intestinal).
- si tiene una enfermedad tiroidea que no se controla con medicamentos.
- si tiene enfermedad hepática avanzada y su hígado no funciona correctamente (p. ej. su piel se ha vuelto amarilla).
- si el paciente es menor de 3 años.
- si el paciente es un niño que hubiera tenido alguna vez enfermedades psiquiátricas graves como depresión grave o pensamientos de intento de suicidio.
- si está infectado con el virus de la hepatitis C y el virus de la inmunodeficiencia humana, y su hígado no funciona correctamente (p. ej., su piel se ha vuelto amarilla).
- si está recibiendo tratamiento con telbivudina, un medicamento para la infección por el virus de la hepatitis B (ver “Otros medicamentos y Pegasys”).
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Pegasys
- si ha padecido un trastorno nervioso o mental grave.
- si ha padecido alguna vez depresión o síntomas relacionados con depresión (p. ej., sentimiento de tristeza, desánimo, etc.).
- si es un adulto que tiene o ha tenido antecedentes de abuso de sustancias (p. ej., alcohol o drogas).
- si tiene psoriasis, esta puede empeorar durante el tratamiento con Pegasys.
- si tiene otros problemas de hígado, aparte de la hepatitis B o C.
- si sufre diabetes o tensión alta, quizá su médico solicite un examen ocular.
- si le han dicho que tiene síndrome VKH.
- si padece una enfermedad tiroidea que no se controle adecuadamente con medicamentos.
- si ha sufrido anemia en alguna ocasión.
- si le han realizado un trasplante de órgano (hígado o riñón) o tiene uno planificado en un futuro próximo.
- si está coinfectado por el virus de la inmunodeficiencia humana (VIH) y en tratamiento con medicamentos anti-VIH.
- si se le ha retirado un tratamiento anterior para la Hepatitis C debido a anemia o a un recuento sanguíneo bajo.
Una vez que haya iniciado el tratamiento con Pegasys, hable con su médico, enfermero o farmacéutico:
- si desarrolla síntomas relacionados con depresión (p. ej., sentimiento de tristeza, desánimo, etc) (ver sección 4).
- si nota algún cambio en la visión.
- si presenta síntomas asociados a un resfriado u otra infección respiratoria (tales como tos, fiebre o cualquier dificultad al respirar).
- si piensa que está contrayendo una infección (como neumonía) ya que cuando esté en tratamiento con Pegasys, puede tener temporalmente un mayor riesgo de contraer infecciones.
- si tiene cualquier signo de hemorragia o presenta hematomas inusuales, consulte con su médico inmediatamente.
- si presenta signos de una reacción alérgica grave (como dificultad al respirar, sonidos sibilantes que se producen al respirar (sibilancias) o urticaria) mientras está en tratamiento con este medicamento, busque inmediatamente ayuda médica.
- si presenta síntomas del síndrome de Vogt-Koyanagi-Harada; combinación de síntomas como rigidez de cuello, dolor de cabeza, pérdida del color de la piel o del pelo, alteraciones oculares (como visión borrosa), y/o alteraciones auditivas (como un pitido en los oídos).
Durante el tratamiento, su médico le tomará muestras de sangre periódicamente para comprobar cambios en sus células sanguíneas blancas (células que combaten infecciones), células sanguíneas rojas (células que transportan oxígeno), plaquetas (células que coagulan la sangre), función hepática, glucosa (niveles de azúcar en sangre) o cambios en otros valores de laboratorio.
Se han comunicado trastornos en dientes y encías que pueden llevar a caída de dientes en pacientes tratados con Pegasys en combinación con ribavirina. Además, la sequedad de boca podría tener efectos perjudiciales sobre los dientes y la mucosa de la boca en tratamientos de larga duración con Pegasys en combinación con ribavirina. Se debe cepillar los dientes adecuadamente dos veces al día y tener revisiones dentales de forma regular. Algunos pacientes, además, pueden sufrir vómitos. Si sufre esta reacción, se debe enjuagar adecuadamente la boca después.
Niños y adolescentes
Indicación para la policitemia vera y la trombocitemia esencial:
Pegasys no se debe administrar a niños y adolescentes debido a que no se dispone de información sobre el uso de Pegasys en este grupo de edad para estas indicaciones.
Indicación para la hepatitis B crónica y hepatitis C crónica:
Pegasys está limitado a niños y adolescentes con hepatitis C crónica a partir de 5 años o niños y adolescentes con hepatitis B crónica a partir de 3 años. Pegasys no se debe administrar a niños menores de 3 años de edad porque contiene alcohol bencílico y puede provocar reacciones tóxicas y reacciones alérgicas en estos niños.
- Si su hijo tiene o ha tenido alguna vez un trastorno psiquiátrico, consulte a su médico, quien hará un seguimiento de su hijo en cuanto a los signos y síntomas de depresión (ver sección 4).
- Mientras esté recibiendo Pegasys, su hijo puede tener un desarrollo y un crecimiento más lento (ver sección 4).
Otros medicamentos y Pegasys
No utilice Pegasys si está tomando telbivudina (ver “No use Pegasys”) debido a que la combinación de estos medicamentos aumenta el riesgo de desarrollar neuropatía periférica (adormecimiento, cosquilleo y/o sensación de ardor en los brazos y/o piernas). Por lo tanto, la combinación de Pegasys con telbivudina está contraindicada. Informe a su médico o farmacéutico si está recibiendo tratamiento con telbivudina.
Comunique a su médico si usted está tomando algún medicamento para el asma ya que puede ser necesario modificar la dosis de la medicación antiasmática.
Pacientes que también tienen infección por VIH: Comunique a su médico si usted está tomando tratamiento anti-VIH. La acidosis láctica y el empeoramiento de la función hepática son efectos adversos asociados con el tratamiento antirretroviral de gran actividad (TARGA), un tratamiento de VIH. Si usted está recibiendo tratamiento (TARGA), la adición de Pegasys + ribavirina puede aumentar el riesgo de acidosis láctica o insuficiencia hepática. Su médico le controlará los signos y síntomas de estas condiciones. Los pacientes que han recibido zidovudina en combinación con ribavirina e interferones alfa pueden tener aumentado el riesgo de desarrollar anemia. Los pacientes que están recibiendo azatioprina en combinación con ribavirina y peginterferón tienen un mayor riesgo de desarrollar trastornos de la sangre graves. Por favor asegúrese también de leer el prospecto de ribavirina.
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Cuando Pegasys se utilice en combinación con ribavirina, tanto los pacientes masculinos como los femeninos deben tener especial precaución en sus relaciones sexuales si existe riesgo de embarazo ya que la ribavirina puede ser muy dañina para el feto.
- Si usted es una mujerpotencialmente fértil y está tomando Pegasys en combinación con ribavirina, usted debe realizarse un test de embarazo y éste debe ser negativo antes de comenzar el tratamiento, cada mes durante el tratamiento y durante 4 meses después de haber finalizado el mismo. Usted debe utilizar métodos anticonceptivos eficaces durante el tratamiento y durante 4 meses después de finalizar el mismo. Usted puede comentar esto con su médico.
- Si usted es un hombrey está tomando Pegasys en combinación con ribavirina, no puede mantener relaciones sexuales con una mujer embarazada a menos que utilice preservativo. De este modo reducirá la probabilidad de que la ribavirina sea liberada al cuerpo de la mujer. Si su pareja no está embarazada, pero es potencialmente fértil, debe realizarse un test de embarazo cada mes durante el tratamiento y durante los 7 meses siguientes a la finalización del mismo. Usted o su pareja deben utilizar métodos anticonceptivos eficaces durante el tiempo en el que usted esté utilizando el tratamiento y durante los 7 meses siguientes a la finalización del tratamiento. Usted puede comentar esto con su médico.
Consulte a su médico o farmacéutico antes de tomar un medicamento. Se desconoce si este producto está presente en la leche humana. Por lo tanto no debe dar el pecho a su bebé si está siendo tratada con Pegasys. En terapia de combinación con ribavirina, preste atención a la información relativa correspondiente a los medicamentos que contienen ribavirina.
Debe consultar también el prospecto de los medicamentos que se usan en combinación con Pegasys.
Conducción y uso de máquinas
No conduzca ni maneje máquinas si se encuentra somnoliento, cansado o confuso mientras esté en tratamiento con Pegasys.
Pegasys contienealcohol bencílico,polisorbato80 y sodio
Este medicamento contiene 5 mg de alcohol bencílico en cada jeringa precargada, lo que equivale a 10 mg/ml.
El alcohol bencílico puede provocar reacciones alérgicas.
El alcohol bencílico se ha relacionado con el riesgo de efectos adversos graves, incluyendo problemas respiratorios (llamado “síndrome del jadeo”) en niños pequeños. Pegasys no debe administrarse a recién nacidos prematuros, recién nacidos a término o niños de hasta 3 años de edad.
Pregunte a su médico o farmacéutico si está embarazada o en periodo de lactancia, o si tiene alguna enfermedad hepática o renal. Esto es porque grandes cantidades de alcohol bencílico pueden acumularse en su cuerpo y causar efectos adversos (llamado “acidosis metabólica”).
Este medicamento contiene 0,025 mg de polisorbato 80 en cada jeringa precargada, que equivale a 0,05 mg/ml. Los polisorbatos pueden causar reacciones alérgicas. Informe a su médico si usted o su hijo tienen alguna alergia conocida.
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis, esto es, esencialmente “exento de sodio”.
3. Cómo usar Pegasys
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Dosificación de Pegasys
Su médico ha establecido la dosis exacta de Pegasys, y le dirá con qué frecuencia debe usarlo. Si es necesario, le cambiará la dosis durante el tratamiento. No sobrepase la dosis que le recomienden.
Policitemia vera y trombocitemia esencial en adultos
Pegasys para tratar la policitemia vera y la trombocitemia esencial se administra solo con la dosis inicial recomendada de 45 microgramos una vez por semana por vía subcutánea y se ajusta habitualmente en incrementos de 45 microgramos una vez al mes hasta un máximo de 180 microgramos una vez por semana por vía subcutánea.
Su médico puede adaptar la dosis y/o prolongar el intervalo de administración.
Hepatitis B y hepatitis C en adultos
Pegasys se utiliza solo (como único tratamiento), tan solo si por algún motivo usted no pudiese tomar ribavirina.
Pegasys, solo o asociado con la ribavirina, suele administrarse en dosis de 180 microgramos una vez por semana. Ver también más adelante los apartados para los tratamientos combinados.
La duración del tratamiento combinado varía de 4 a 18 meses dependiendo del tipo de virus con el que usted esté infectado, de la respuesta durante el tratamiento y de si usted ha sido tratado anteriormente.
Por favor consulte a su médico y siga la duración de tratamiento recomendada.
La inyección de Pegasys normalmente se administra a la hora de acostarse.
Uso en niños y adolescentes
Hepatitis B (a partir de 3años de edad) y hepatitis C (a partir de 5años de edad)
Su médico ha determinado la dosis exacta de Pegasys para su hijo y le informará cada cuánto tiene que usarlo. La dosis habitual de Pegasys está basada en la altura y el peso de su hijo. Si fuera necesario, se podría cambiar la dosis durante el tratamiento. Para niños y adolescentes se recomienda utilizar Pegasys jeringas precargadas, debido a que estas permiten ajustar la dosis. No sobrepase la dosis recomendada.
La duración del tratamiento combinado en niños con hepatitis C crónica varía de 6 a 12 meses, dependiendo del tipo de virus con el cual esté infectado su hijo y de su respuesta al tratamiento. La duración del tratamiento con Pegasys en hepatitis B crónica es de 48 semanas. Consulte a su médico y siga la duración del tratamiento recomendada. Normalmente, la inyección de Pegasys se pone a la hora de acostarse.
Pegasys se administra por vía subcutánea (debajo de la piel). Esto significa que Pegasys se inyecta con una aguja corta en el tejido graso bajo la piel del abdomen o del muslo. Si usted se va a inyectar este medicamento por sí mismo, debe ser instruido sobre cómo hacerlo. Las instrucciones pormenorizadas se adjuntan al final del prospecto (ver “Cómo inyectar Pegasys”).
Use Pegasys exactamente como se lo haya dicho su médico y durante el tiempo que le haya indicado. Si estima que la acción de Pegasys es demasiado fuerte o débil, indíqueselo al médico o farmacéutico.
Tratamiento combinado con ribavirina en hepatitis C crónica(todos los pacientes a partir de 5años de edad)
En el caso de tratamiento de combinación de Pegasys y ribavirina, por favor siga el régimen de dosificación recomendado por su médico.
Tratamiento combinado con otros medicamentos en hepatitis C crónica(todos los pacientes a partir de 5años de edad)
En el caso de tratamiento de combinación con Pegasys, por favor siga el régimen de dosificación recomendado por su médico y debe consultar también el prospecto de los medicamentos que se usan en combinación con Pegasys.
Si usa más Pegasys del que debe
Contacte con su médico o farmacéutico tan pronto como le sea posible.
Si olvidó usar Pegasys
Si se percata de que ha olvidado la inyección 1 o 2 días después de lo previsto,inyéctese la dosis recomendada lo antes posible. Continúe con la siguiente inyección siguiendo el calendario previsto.
Si se percata de que ha olvidado la inyección 3 a 5 días después de lo previsto, inyecte la dosis recomendada lo antes posible y pase a inyectar las dosis siguientes en intervalos de 5 días hasta que recupere el ritmo semanal previsto.
Ejemplo: usted se inyecta Pegasys todos los lunes. Sin embargo, el viernes se da cuenta de que se olvidó la inyección correspondiente al lunes anterior (retraso de 4 días). Debe inyectarse la dosis regular de forma inmediata el mismo viernes y la siguiente, el miércoles de la semana próxima (5 días después de la dosis del viernes). La próxima inyección tendrá lugar el lunes, 5 días después del miércoles. Ya ha recuperado ahora el ritmo semanal antiguo y podrá continuar inyectándose todos los lunes.
Si se percata de que ha olvidado la inyección 6 días después de lo previsto,debe esperar e inyectarse la dosis al día siguiente para mantener el ritmo habitual.
Consulte a su médico o farmacéutico si necesita información más detallada, en caso de que olvide alguna dosis de Pegasys.
No tome una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Algunas personas pueden sufrir depresión cuando son tratadas con Pegasys solo o en combinación con ribavirina, y en algunos casos han tenido pensamientos suicidas o comportamiento agresivo (a veces dirigido hacia otras personas como la idea de atentar contra la vida de los demás). De hecho algunos pacientes se han suicidado. Asegúrese de buscar cuidados de emergencia si siente que se está deprimiendo o tiene pensamientos suicidas o cambios en su comportamiento. Quizá pueda considerar la posibilidad de pedirle a un familiar o amigo cercano que le ayude a estar atento a señales de depresión o cambios en su comportamiento. |
Crecimiento y desarrollo (niños y adolescentes) Algunos niños y adolescentes tratados con Pegasys para la hepatitis B crónica durante 48 semanas no crecieron ni aumentaron de peso según lo esperado para su edad. Se desconoce todavía si volverán a su altura y peso previsto después de completar el tratamiento. Durante el año de tratamiento con Pegasys en combinación con ribavirina, algunos niños y adolescentes con hepatitis C crónica no crecieron ni ganaron tanto peso como el esperado. La mayoría de los niños alcanzaron la altura esperada durante los dos años después de acabar el tratamiento, y el resto de los niños dentro de los seis años después de terminar el tratamiento, es posible que Pegasys pueda afectar a su altura adulta final. |
Hable inmediatamente con su médico si tiene alguna de estas reacciones adversas: dolor de pecho grave, tos persistente, latidos irregulares del corazón, problemas al respirar, confusión, depresión, dolor de estómago grave, sangre en heces (o heces alquitranosas negras), sangrado nasal grave, fiebre o escalofríos, problemas de la vista. Estos efectos adversos pueden ser graves y usted puede necesitar atención médica urgente.
Los efectos adversos muy frecuentes de Pegasys en combinación con ribavirina (pueden afectar a más de 1 de cada 10 personas) son:
Trastornos del metabolismo: pérdida de apetito
Trastornos psiquiátricos y del sistema nervioso: sentimiento depresivo (sentimiento de tristeza, bajo estado de ánimo, pesimismo), ansiedad, insomnio, dolor de cabeza, dificultad para concentrarse y mareos
Trastornos respiratorios: tos, dificultad al respirar
Trastornos digestivos: diarrea, náuseas, dolor abdominal
Trastornos de la piel: caída de pelo y reacciones de la piel (incluyendo picor, dermatitis y sequedad de piel)
Trastornos musculares y óseos: dolor en las articulaciones y dolor muscular
Trastornos generales: fiebre, debilidad, cansancio, temblores, escalofríos, dolor, irritación en el lugar de la inyección e irritabilidad (fácilmente irritable)
Los efectos adversos frecuentes de Pegasys en combinación con ribavirina (pueden afectar a hasta 1 de cada 10 personas) son:
Infecciones: infecciones por hongos, virus y bacterias. Infecciones de las vías respiratorias altas, bronquitis, infecciones por hongos en la boca y herpes (infección viral común y recurrente que afecta a labios y boca)
Trastornos de la sangre: bajo recuento de plaquetas (afecta a la capacidad de coagulación), anemia (bajo recuento de glóbulos rojos) e inflamación de los ganglios linfáticos
Trastornos del sistema hormonal: alta y baja actividad de la glándula tiroidea
Trastornos psiquiátricos y del sistema nervioso: cambios de humor/emocionales, agresividad, nerviosismo, disminución del deseo sexual, pérdida de memoria, desmayo, disminución del tono muscular, migraña, entumecimiento, hormigueo, sensación de calor, temblor, alteración del sentido del gusto, pesadillas, somnolencia
Trastornos oculares: visión borrosa, dolor de ojos, inflamación de los ojos y sequedad de ojos
Trastorno del oído: dolor de oídos
Trastornos del corazón y de los vasos sanguíneos: latido rápido del corazón, palpitaciones, inflamación de las extremidades, rubor
Trastornos respiratorios: dificultad al respirar cuando se hace ejercicio, hemorragias nasales, inflamación de la nariz y garganta, infecciones de la nariz y de los senos (cavidades llenas de aire que se encuentran en los huesos de la cabeza y la cara), secreción nasal, dolor de garganta
Trastornos digestivos: vómitos, indigestión, dificultad al tragar, úlceras en la boca, sangrado de encías, inflamación de la lengua y de la boca, flatulencia (exceso de aire o gases), sequedad de boca y pérdida de peso
Trastornos de la piel: sarpullido, aumento de la sudoración, psoriasis, enrojecimiento y picor de la piel (urticaria), eczema, sensibilidad a la luz, sudores nocturnos
Trastornos musculares y óseos: dolor de espalda, inflamación de las articulaciones, debilidad muscular, dolor de huesos, dolor de cuello, dolor muscular, calambres musculares
Trastornos del aparato reproductor: impotencia (incapacidad para mantener una erección)
Trastornos generales: dolor torácico, síntomas pseudogripales, malestar, letargia, sofocos, sed
Los efectos adversos poco frecuentes de Pegasys en combinación con ribavirina (pueden afectar a hasta 1 de cada 100 personas) son:
Infecciones: infección pulmonar, infecciones de la piel
Neoplasias benignas y malignas: tumor hepático
Trastornos del sistema inmunológico: sarcoidosis (áreas del tejido inflamadas por todo el cuerpo), inflamación de tiroides
Trastornos del sistema hormonal: diabetes (niveles altos de azúcar en sangre)
Trastornos del metabolismo: deshidratación
Trastornos psiquiátricos y del sistema nervioso: pensamientos suicidas, alucinaciones, neuropatía periférica (trastornos de los nervios que afectan a las extremidades)
Trastornos oculares: hemorragia de la retina (parte posterior del ojo)
Trastornos del oído: pérdida de audición
Trastornos del corazón y de los vasos sanguíneos: tensión alta
Trastornos respiratorios: sonidos sibilantes que se producen al respirar (sibilancias)
Trastornos digestivos: hemorragia gastrointestinal
Trastornos hepáticos: disfunción hepática
Los efectos adversos raros de Pegasys en combinación con ribavirina (pueden afectar a hasta 1 de cada 1 000 personas) son:
Infecciones: infección del corazón, infección del oído externo
Trastornos de la sangre: disminución grave de células sanguíneas rojas, células sanguíneas blancas y plaquetas
Trastornos del sistema inmunológico: reacciones alérgicas graves, lupus eritematoso sistémico (el cuerpo ataca sus propias células), artritis reumatoide (una enfermedad autoinmune)
Trastornos del sistema hormonal: cetoacidosis diabética, una complicación de la diabetes no controlada
Trastornos psiquiátricos y del sistema nervioso: suicidio, trastornos psicóticos (problemas graves de personalidad y deterioro en el normal funcionamiento social), coma (inconsciencia profunda y prolongada), convulsiones, parálisis facial (debilidad de los músculos faciales)
Trastornos oculares: inflamación del nervio óptico, inflamación de la retina, úlcera en la córnea
Trastornos del corazón y de los vasos sanguíneos: ataque cardiaco, fallo cardiaco, dolor cardiaco, latido rápido del corazón, alteración del ritmo cardiaco o inflamación del revestimiento del corazón y del músculo cardiaco, hemorragia cerebral e inflamación en los vasos
Trastornos respiratorios: neumonía intersticial (inflamación de los pulmones incluyendo desenlace mortal), coágulos de sangre en los pulmones
Trastornos digestivos: úlcera gástrica, inflamación del páncreas
Trastornos hepáticos: fallo hepático, inflamación de los conductos biliares, hígado graso
Trastornos musculares y óseos: inflamación de los músculos
Trastornos del riñón: fallo renal
Lesiones traumáticas o intoxicaciones: sobredosis
Los efectos adversos muy raros de Pegasys en combinación con ribavirina (pueden afectar a hasta 1 de cada 10 000 personas) son:
Trastornos de la sangre: anemia aplásica (fallo de la médula ósea en la producción de células sanguíneas rojas, células sanguíneas blancas y plaquetas)
Trastornos del sistema inmunológico: púrpura trombocitopénica idiopática o trombótica (aumento de hematomas, sangrado, disminución de plaquetas, anemia y debilidad extrema)
Trastornos oculares: pérdida de visión
Trastornos de la piel: necrólisis epidérmica tóxica/ Síndrome de Stevens-Johnson/ eritema multiforme (un rango de sarpullido con diversos grados de severidad que incluye la muerte, que pueden ir asociadas con ampollas en la boca, nariz, ojos y otras membranas de la mucosa y desprendimiento de la zona afectada de la piel), angioedema (inflamación de la piel y mucosas)
Efectos adversos de frecuencia desconocida:
Trastornos de la sangre: aplasia eritrocitaria pura (un tipo grave de anemia en el que se disminuye o anula la producción de células sanguíneas rojas), que puede dar lugar a síntomas tales como sensación de estar muy cansado y sin energía
Trastornos del sistema inmunológico: enfermedad de Vogt Koyanagi Harada, una enfermedad rara caracterizada por la pérdida de visión, oído y pigmentación de la piel; rechazo a trasplante de hígado y riñón
Trastornos psiquiátricos y del sistema nervioso: manía (episodios de exaltación exagerada del estado de ánimo) y trastornos bipolares (episodios de exaltación exagerada del estado de ánimo alternando con tristeza y desesperanza); pensamientos de amenaza contra la vida de los demás, ictus (falta de riego sanguíneo en el cerebro)
Trastornos oculares: forma rara de desprendimiento de retina con líquido en la retina
Trastornos del corazón y de los vasos sanguíneos: isquemia periférica (aporte insuficiente de sangre a las extremidades)
Trastornos digestivos: colitis isquémica (aporte sanguíneo al intestino insuficiente), cambios en el color de la lengua
Trastornos musculares y óseos: daño muscular grave y dolor
La hipertensión arterial pulmonar es una enfermedad en la que se produce un gran estrechamiento de los vasos sanguíneos de los pulmones provocando un aumento de la presión en los vasos sanguíneos que transportan la sangre del corazón a los pulmones. Esto se puede producir especialmente en pacientes con factores de riesgo como la infección por el VIH o problemas graves de hígado (cirrosis). Los episodios se notificaron en distintos momentos, incluso varios meses después del inicio del tratamiento con Pegasys.
Si solo se administra Pegasys en pacientes con hepatitis B o C, la probabilidad de estos efectos se reduce.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Pegasys
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2°C y 8°C). No congelar.
Mantener la jeringa precargada en el embalaje original para protegerla de la luz.
No utilice este medicamento si observa que la jeringa o el envase de la aguja están dañados, si la solución está turbia, tiene partículas flotando o si tiene otro color que no sea entre incoloro a amarillo claro.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Pegasys
- El principio activo es el peginterferón alfa-2a. Cada jeringa precargada de 0,5 ml de solución contiene 90, 135 o 180 microgramos de peginterferón alfa-2a.
- Los demás componentes son cloruro sódico, polisorbato 80, alcohol bencílico, acetato sódico, ácido acético y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Pegasys se presenta en forma de solución inyectable contenida en una jeringa precargada (0,5 ml) con una aguja de inyección separada.
Pegasys 90 microgramos solución inyectable en jeringa precargada
La jeringa tiene marcas de graduación que corresponden a 90 microgramos (µg), 65 µg, 45 µg, 30 µg, 20 µg y 10 µg. Está disponible en envases de 1 jeringa precargada.
Pegasys 135 microgramos solución inyectable en jeringa precargada
La jeringa tiene marcas de graduación que corresponden a 135 microgramos (µg), 90 µg y 45 µg. Está disponible en envases de 1, 4 o envase múltiple que contiene 12 (2 envases de 6) jeringas precargadas. Puede que solamente estén comercializados algunos tamaños de envases.
Pegasys 180 microgramos solución inyectable en jeringa precargada
La jeringa tiene marcas de graduación que corresponden a 180 microgramos (µg), 135 µg y 90 µg. Está disponible en envases de 1, 4 o envase múltiple que contiene 12 (2 envases de 6) jeringas precargadas. Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
pharmaand GmbH
Taborstrasse 1
1020 Wien
Austria
Responsable de la fabricación
Loba biotech GmbH
Fehrgasse 7
2401 Fischamend
Austria
Fecha de la última revisión de esteprospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu.
Cómo inyectar Pegasys
Las instrucciones siguientes le explican cómo usar las jeringas precargadas de Pegasys para inyectarse usted mismo o a su hijo. Por favor, lea las instrucciones cuidadosamente y sígalas paso a paso. Su médico u otro personal sanitario le dará instrucciones sobre cómo administrar las inyecciones.
Preparación inicial
Lávese las manos cuidadosamente antes de manejar cualquiera de los materiales.
Tenga disponible todo lo necesario antes de comenzar:
Incluido en el envase:
- una jeringa precargada de Pegasys
- una aguja para inyección
No incluido en el envase:
- un algodón para limpiar
- venda pequeña o gasa estéril
- una tirita adhesiva
- un envase para material de desecho
Preparación de la jeringa y de la aguja para la inyección
- Quite la tapa protectora que cubre el final de la aguja (1-2).
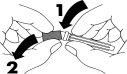
- Quite la tapa de goma de la jeringa (3). No toque la punta de la jeringa.
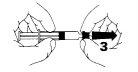
- Coloque firmemente la aguja en la punta de la jeringa (4).
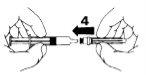
- Quite la protección de la aguja (5).
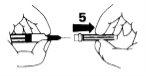
- Para eliminar las burbujas de aire de la jeringa, sosténgala en posición vertical con la aguja hacia arriba. Golpee con el dedo varias veces la jeringa para que las burbujas vayan hacia la parte superior del líquido. Empuje el émbolo lentamente hasta llegar a la dosis correcta, donde el borde del émbolo entra en contacto con la jeringa. Vuelva a poner la protección de la aguja y coloque la jeringa en posición horizontal hasta que esté listo para usarla.
- Deje que la solución alcance la temperatura ambiente antes de la inyección o bien caliente la jeringa sosteniéndola entre sus manos.
- Inspeccione visualmente la solución antes de su administración: no la use si presenta cambios de color o si observa que tiene partículas.
Ahora está listo para inyectar la dosis.
Inyectar la solución
- Seleccione el lugar del abdomen o el muslo para la inyección (excepto la zona del ombligo o de la cintura). Cambie el lugar de inyección cada vez que se lo administre.
- Limpie y desinfecte la piel en la zona donde vaya a inyectarse con un algodón.
- Espere que se seque la zona.
- Quite la protección de la aguja.
- Con una mano pellizque la piel y, con la otra mano, coja la jeringa como si fuese un bolígrafo.
- Inserte la aguja totalmente con un ángulo de 45º a 90º en la piel que tiene cogida con la otra mano (6).
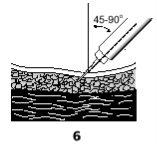
- Inyecte la solución empujando el émbolo hacia abajo desde la graduación apropiada.
- Saque la aguja de la piel.
- Si es necesario, presione el lugar donde se ha inyectado con un pequeño vendaje o gasa estéril durante unos segundos.
No masajee el lugar de la inyección. Si sangrara, cúbralo con una tirita adhesiva.
Eliminación de los materiales de inyección
La jeringa, aguja y todos los materiales de inyección son de un solo uso y deben ser desechados tras la inyección. Elimine la jeringa y aguja de forma segura en un contenedor cerrado. Pida a su médico, hospital o farmacéutico un contenedor apropiado.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a PEGASYS, 180 microgramos, SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 90 mcg / 0,5 mlPrincipio activo: Peginterferon alfa- 2aFabricante: Pharmaand GmbhRequiere recetaForma farmacéutica: INYECTABLE, DesconocidaPrincipio activo: Peginterferon alfa- 2aFabricante: Pharmaand GmbhRequiere recetaForma farmacéutica: INYECTABLE, DesconocidaPrincipio activo: Peginterferon alfa- 2aFabricante: Pharmaand GmbhRequiere receta
Médicos online para PEGASYS, 180 microgramos, SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de PEGASYS, 180 microgramos, SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















