
NORDIMET 12,5 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar NORDIMET 12,5 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Nordimet 7,5 mg solución inyectable en pluma precargada
Nordimet 10 mg solución inyectable en pluma precargada
Nordimet 12,5 mg solución inyectable en pluma precargada
Nordimet 15 mg solución inyectable en pluma precargada
Nordimet 17,5 mg solución inyectable en pluma precargada
Nordimet 20 mg solución inyectable en pluma precargada
Nordimet 22,5 mg solución inyectable en pluma precargada
Nordimet 25 mg solución inyectable en pluma precargada
metotrexato
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Nordimet y para qué se utiliza
- Qué necesita saber antes de empezar a usar Nordimet
- Cómo usar Nordimet
- Posibles efectos adversos
- Conservación de Nordimet
- Contenido del envase e información adicional
1. Qué es Nordimet y para qué se utiliza
Nordimet contiene el principio activo metotrexato, el cual:
- reduce la inflamación o hinchazón, y
- reduce la actividad del sistema inmunitario (el mecanismo de defensa propio del organismo). Se ha relacionado un sistema inmunitario hiperactivo con enfermedades inflamatorias.
Nordimet es un medicamento utilizado para tratar varias enfermedades inflamatorias:
- Artritis reumatoide activa en adultos. La artritis reumatoide activa es una enfermedad inflamatoria que afecta a las articulaciones.
- Artritis idiopática juvenil activa grave en cinco o más articulaciones (por ello este trastorno recibe el nombre de poliartrítica), en pacientes que han presentado una respuesta inadecuada a los fármacos antiinflamatorios no esteroideos (AINEs).
- Psoriasis en placas de moderada a grave en adultos candidatos a tratamiento sistémico, así como en la psoriasis grave que también afecta a las articulaciones (artritis psoriásica) en pacientes adultos.
- Inducción de la remisión en adultos con enfermedad de Crohn moderada dependiente de corticosteroides, en combinación con corticosteroides.
- Mantenimiento de la remisión de la enfermedad de Crohn en adultos que han respondido al metotrexato, en monoterapia.
2. Qué necesita saber antes de empezar a usar Nordimet
No use Nordimet si:
- es alérgico al metotrexato o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- padece una nefropatía grave (su médico podrá decirle si tiene una nefropatía grave).
- padece una enfermedad hepática grave (su médico podrá decirle si tiene una enfermedad hepática grave).
- padece trastornos del sistema hematopoyético.
- realiza un consumo de alcohol elevado.
- tiene un sistema inmunitario debilitado.
- padece una infección grave o existente, por ejemplo, tuberculosis o VIH.
- tiene úlceras gastrointestinales.
- está embarazada o en período de lactancia (ver la sección “Embarazo, lactancia y fertilidad”).
- recibe vacunas con microorganismos vivos de forma simultánea.
Advertencias y precauciones
Se ha notificado con metotrexato hemorragia pulmonar aguda en pacientes con enfermedad reumatológica subyacente. Si observa sangre al escupir o toser, se debe poner en contacto de forma inmediata con su médico.
Puede producirse un aumento de tamaño de los ganglios linfáticos (linfoma), en cuyo caso hay que interrumpir el tratamiento.
La diarrea puede ser un efecto tóxico de Nordimet y requiere la interrupción del tratamiento.
Si padece diarrea, hable con su médico.
Se han notificado ciertos trastornos cerebrales (encefalopatía/leucoencefalopatía) en pacientes con cáncer que reciben metotrexato. Estos efectos secundarios no se pueden descartar cuando se utiliza metotrexato para tratar otras enfermedades.
Si usted, su pareja o su cuidador notan la aparición o un empeoramiento de síntomas neurológicos, como debilidad muscular general, alteraciones de la visión, cambios en el pensamiento, la memoria y la orientación que generan confusión y cambios en la personalidad, contacte con su médico inmediatamente porque estos pueden ser síntomas de una infección cerebral grave muy rara denominada leucoencefalopatía multifocal progresiva (LMP).
El metotrexato puede hacer que la piel sea más sensible a la luz solar. Evite el sol intenso y no utilice camas de bronceado ni lámparas ultravioletas sin consejo médico. Para proteger la piel del sol intenso, lleve ropa adecuada o utilice un protector solar con un factor de protección alto.
Advertencia importante sobre la administración de Nordimet
Solo se debe utilizar metotrexato para el tratamiento de enfermedades reumáticas, de la piel y de la enfermedad de Crohn una vez por semana. La administración incorrecta de metotrexato puede producir efectos adversos graves que pueden ser mortales. Lea detenidamente la sección 3 de este prospecto.
Consulte a su médico antes de empezar a usar Nordimet si:
- tiene diabetes mellitus y recibe tratamiento con insulina;
- padece infecciones prolongadas inactivas (por ejemplo, tuberculosis, hepatitis B o C, herpes zóster);
- padece/ha padecido alguna enfermedad hepática o nefropatía;
- tiene problemas con la función pulmonar;
- tiene un sobrepeso grave;
- presenta una acumulación anómala de líquido en el abdomen o en la cavidad entre los pulmones y la pared torácica (ascitis, derrames pleurales);
- está deshidratado o tiene un trastorno que produce deshidratación (por ejemplo, deshidratación como consecuencia de vómitos, diarrea o inflamación de la boca y los labios).
Si ha experimentado problemas en la piel después de radioterapia (dermatitis inducida por radiación) o quemaduras en la piel, estas alteraciones pueden reaparecer al tomar Nordimet.
Niños, adolescentes y pacientes de edad avanzada
Las instrucciones sobre la dosis dependen del peso corporal del paciente.
No se recomienda el uso en menores de 3 años debido a una experiencia insuficiente en el uso de este medicamento en este grupo de edad.
Los niños, adolescentes y los pacientes de edad avanzada tratados con Nordimet se deben someter a una estrecha vigilancia médica para identificar posibles efectos adversos lo antes posible.
La dosis para pacientes de edad avanzada se debe disminuir debido a una disminución de las funciones hepática y renal relacionada con la edad.
Medidas de precaución especiales para el tratamiento con Nordimet
El metotrexato afecta temporalmente al semen y a la producción de óvulos. El metotrexato puede causar abortos y defectos de nacimiento graves. Si usted es mujer, debe evitar tener un bebé si está recibiendo metotrexato en ese momento y durante al menos 6 meses después de finalizar el tratamiento con metotrexato. Si usted es hombre, debe evitar tener un hijo si está recibiendo metotrexato en ese momento y durante al menos 3 meses después de finalizar el tratamiento.
Consulte también la sección “Embarazo, lactancia y fertilidad”.
Los cambios en la piel causados por la psoriasis pueden empeorar durante el tratamiento con Nordimet en caso de exposición a irradiación ultravioleta.
Exploraciones de seguimiento y precauciones recomendadas
Incluso cuando se usa metotrexato en dosis bajas, pueden aparecer efectos adversos graves. Para detectarlos a tiempo, su médico llevará a cabo exploraciones de control y pruebas de laboratorio.
Antes de iniciar el tratamiento:
Antes de iniciar el tratamiento, se le hará un análisis de sangre para determinar si tiene suficientes células sanguíneas. También se le hará un análisis de sangre para comprobar la función hepática y descubrir si padece hepatitis. Además, también se comprobarán los niveles de albúmina sérica (una proteína sanguínea), el estado de la hepatitis (infección hepática) y la función renal. El médico también puede decidir realizar otras pruebas hepáticas, que pueden consistir en la obtención de imágenes del hígado o la toma de una pequeña muestra de tejido del hígado para realizar una exploración más exhaustiva. También es posible que el médico compruebe si padece tuberculosis y le realice una radiografía torácica o una prueba de la función pulmonar.
Durante el tratamiento:
El médico podrá realizar las siguientes exploraciones:
- Exploración de la cavidad oral y la faringe para detectar cambios en la mucosa, como inflamación o úlceras.
- Análisis de sangre/hemograma con recuento de células sanguíneas y medición de los niveles séricos de metotrexato.
- Análisis de sangre para controlar la función hepática.
- Pruebas de imagen para controlar el estado del hígado.
- Obtención de una pequeña muestra de tejido del hígado para realizar una exploración más exhaustiva.
- Análisis de sangre para controlar la función renal.
- Control de las vías respiratorias y, si fuera necesario, prueba de función pulmonar.
Es muy importante que acuda a realizarse estas exploraciones programadas.
Si los resultados de algunas de estas pruebas son anómalos, su médico ajustará el tratamiento de manera acorde.
Otros medicamentos y Nordimet
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Es especialmente importante que informe a su médico si está tomando:
- otros tratamientos para la artritis reumatoide o psoriasis como leflunomida, sulfasalazina (un medicamento que, además de emplearse en la artritis y la psoriasis, también se utiliza para el tratamiento de la colitis ulcerosa), ácido acetilsalicílico, fenilbutazona o amidopirina;
- ciclosporina (para suprimir el sistema inmunitario);
- azatioprina (se utiliza para evitar el rechazo después de un trasplante de órganos);
- retinoides (se utilizan para tratar la psoriasis y otros trastornos de la piel);
- medicamentos anticonvulsivantes (se utilizan para prevenir ataques), como fenitoína, valproato o carbamazepina;
- tratamientos para el cáncer;
- barbitúricos (inyección para dormir);
- tranquilizantes;
- anticonceptivos orales;
- probenecid (se utiliza para el tratamiento de la gota);
- antibióticos (por ejemplo, penicilina, glucopéptidos, trimetoprim-sulfametoxazol, sulfonamidas, ciprofloxacina, cefalotina, tetraciclinas, cloranfenicol);
- pirimetamina (se utiliza para prevenir y tratar la malaria);
- preparados vitamínicos con ácido fólico;
- inhibidores de bomba de protones (medicamentos que reducen la producción de ácido gástrico y que se utilizan para tratar el ardor de estómago o úlceras graves), como omeprazol o pantoprazol;
- teofilina (se utiliza para tratar el asma);
- colestiramina (se utiliza para tratar el colesterol alto, el prurito o la diarrea);
- AINEs, antiinflamatorios no esteroideos (se utilizan para tratar el dolor o la inflamación);
- ácido p-aminobenzoico (se utiliza para tratar los trastornos cutáneos);
- cualquier vacuna con microorganismos vivos (se debe evitar), como vacunas para el sarampión, las paperas, la gripe y la fiebre amarilla;
- metamizol (sinónimos: novaminsulfon y dipirona) (medicamento para el dolor intenso y/o la fiebre);
- óxido nitroso (un gas que se utiliza en la anestesia general)
Nordimet con alimentos, bebidas y alcohol
Durante el tratamiento con Nordimet, debe evitar el consumo de alcohol y el consumo excesivo de café, refrescos que contengan cafeína y té negro, ya que pueden aumentar los efectos adversos o interferir en la eficacia de Nordimet. Asimismo, asegúrese de beber líquidos en abundancia durante el tratamiento con Nordimet, ya que la deshidratación (reducción del agua corporal) puede aumentar la toxicidad de Nordimet.
Embarazo, lactancia y fertilidad
Si está embarazada o en período de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Embarazo
No utilice Nordimet durante el embarazo o si está intentando quedarse embarazada. El metotrexato puede causar anomalías congénitas, perjudicar al feto o producir abortos. Se asocia a malformaciones de cráneo, cara, corazón y vasos sanguíneos, cerebro y extremidades. Por ello, es muy importante que no se administre metotrexato a mujeres embarazadas o que tengan previsto quedarse embarazadas. En las mujeres en edad fértil, debe excluirse cualquier posibilidad de embarazo con las medidas adecuadas, por ejemplo, una prueba de embarazo, antes de iniciar el tratamiento. Debe evitar quedarse embarazada mientras toma metotrexato y durante al menos 6 meses después de finalizar el tratamiento mediante el uso de métodos anticonceptivos fiables durante este tiempo (consulte también la sección “Advertencias y precauciones”).
Si se queda embarazada durante el tratamiento o sospecha que podría estar embarazada, hable con su médico tan pronto como sea posible. Deben informarle sobre el riesgo de efectos perjudiciales para el feto durante el tratamiento.
Si desea quedarse embarazada debe consultar a su médico, que podría remitirle a un especialista antes del inicio previsto de tratamiento.
Lactancia
No dé el pecho durante el tratamiento, porque el metotrexato pasa a la leche materna. Si el médico considera que el tratamiento con metotrexato es absolutamente necesario durante el período de lactancia, debe dejar de dar el pecho.
Fertilidad masculina
Las evidencias disponibles no indican riesgo aumentado de malformaciones o abortos si el padre toma menos de 30 mg/semana de metotrexato. No obstante, no es posible descartar por completo un cierto riesgo. El metotrexato puede ser genotóxico. Esto significa que el medicamento puede causar mutaciones genéticas. El metotrexato puede afectar a la producción de espermatozoides y causar anomalías congénitas. Por esta razón, debería evitar engendrar un hijo o donar semen mientras toma metotrexato y durante al menos 3 meses después de la finalización del tratamiento.
Conducción y uso de máquinas
Pueden producirse efectos adversos que afecten al sistema nervioso central, como fatiga y mareos, durante el tratamiento con Nordimet. En algunos casos, la capacidad para conducir vehículos y/o utilizar máquinas puede verse reducida. Si siente fatiga o mareos no debe conducir ni usar máquinas.
Nordimet contiene sodio
Este medicamento contiene menos de 1 mmol (23 mg) de sodio por dosis, esto es, esencialmente “exento de sodio”.
3. Cómo usar Nordimet
Advertencia importante sobre la dosis de Nordimet
Utilice Nordimet solo una vez por semanapara el tratamiento de la artritis reumatoide, la artritis idiopática juvenil activa, la psoriasis, la artritis psoriásica y la enfermedad de Crohn, que requiere una administración semanal. El uso excesivo de Nordimet puede ser mortal. Lea la sección 3 de este prospecto con mucha atención. Si tiene alguna duda, consulte a su médico o farmacéutico antes de tomar este medicamento.
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Nordimet se administra solo una vez por semana. Usted y su médico pueden acordar qué día de la semana recibirá la inyección cada semana.
La administración incorrecta de Nordimet puede producir efectos adversos graves que pueden ser mortales.
La dosis recomendada es:
Dosis en pacientes con artritis reumatoide
La dosis inicial recomendada es de 7,5 mg de metotrexato una vez por semana.
El médico puede aumentar la dosis si la dosis utilizada no es eficaz, pero se tolera bien. La dosis semanal media es de 15-20 mg. Generalmente, no se debe superar una dosis semanal de 25 mg. Cuando Nordimet empiece a funcionar, el médico podrá reducir la dosis gradualmente hasta la dosis más baja de mantenimiento eficaz posible.
Generalmente, se prevé la mejora de los síntomas después de 4-8 semanas de tratamiento. Los síntomas pueden volver si el tratamiento con Nordimet se interrumpe.
Uso en adultos con psoriasis en placas de moderada a grave o artritis psoriásica grave
Su médico le administrará una sola dosis de prueba de 5-10 mg con el fin de evaluar los posibles efectos adversos.
Si la dosis de prueba se tolera bien, se continuará el tratamiento al cabo de una semana con una dosis aproximada de 7,5 mg.
Generalmente, se puede prever una respuesta al tratamiento después de aproximadamente 2-6 semanas. En función de los efectos del tratamiento y los resultados de los análisis de sangre y orina, el tratamiento continuará o se interrumpirá.
Dosis en pacientes adultos con enfermedad de Crohn
Su médico empezará el tratamiento con una dosis semanal de 25 mg. Generalmente, se puede prever una respuesta al tratamiento después de 8-12 semanas. En función de los efectos del tratamiento, llegado el momento, su médico podrá decidir reducir la dosis a 15 mg por semana.
Uso en niños y adolescentes menores de 16 años con formas poliartríticas de artritis idiopática juvenil
El médico calculará la dosis necesaria a partir de la superficie corporal del niño (m2), y la dosis se expresa en mg/m2.
El uso en niños < 3 años de edad no está recomendado, puesto que no se dispone de experiencia suficiente en este grupo de edad.
Método y duración de la administración
Nordimet se administra mediante una inyección debajo de la piel (por vía subcutánea). Se debe inyectar una vez por semana y se recomienda inyectar Nordimet siempre el mismo día de la semana.
Al principio de su tratamiento, puede que le inyecte Nordimet personal médico. No obstante, su médico puede decidir que sea usted mismo quien aprenda a inyectarse Nordimet. Usted recibirá la formación adecuada para hacer esto. En ninguna circunstancia debe intentar autoinyectarse, a no ser que le hayan enseñado cómo hacerlo.
La duración del tratamiento será determinada por el médico responsable del tratamiento. El tratamiento de la artritis reumatoide, la artritis idiopática juvenil, la psoriasis en placas, la artritis psoriásica y la enfermedad de Crohn con Nordimet es un tratamiento a largo plazo.
Información sobre cómo inyectarse Nordimet usted mismo
Si tiene dificultades para manipular la pluma, consulte a su médico o farmacéutico. No intente autoinyectarse si no le han enseñado cómo hacerlo. Si no está seguro, consulte a su médico o enfermero inmediatamente.
Antes de autoinyectarse Nordimet
- Compruebe la fecha de caducidad del medicamento. No lo use si ha caducado.
- Compruebe que la pluma no esté dañada y que el medicamento sea una solución transparente y amarillenta. De lo contrario, utilice otra pluma.
- Examine el lugar de la última inyección para comprobar si causó enrojecimiento, cambios del color de la piel, hinchazón o supuración o si todavía le duele. En ese caso, hable con su médico o enfermero.
- Decida dónde inyectará el medicamento. Cambie el lugar en el que se autoinyecta el medicamento cada vez.
Instrucciones sobre cómo autoinyectarse Nordimet
- Lávese bien las manos con agua y jabón.
- Siéntese o recuéstese en una posición cómoda y relajada. Asegúrese de que puede ver la zona de la piel en la que efectuará la inyección.
- La pluma está precargada y lista para usar. Inspeccione visualmente la pluma. Debe poder ver un líquido amarillo a través de la ventana de visualización. Es posible que vea una pequeña burbuja de aire, esta no afectará a la inyección y no le causará ningún daño.
Puede que aparezca una gota en la punta de la aguja. Esto es normal.
- Elija un lugar de inyección y límpielo con la torunda impregnada con alcohol que va incluida. Tarda entre 30 y 60 segundos en hacer efecto. Se consideran zonas adecuadas para la inyección la piel de la parte delantera de la pared abdominal y la piel de la parte delantera del muslo.
- Mientras sujeta el cuerpo de la pluma, retire el tapón protector verde tirando de él suavemente y directamente hacia fuera de la unidad. No lo gire ni lo doble.
Después de retirar el tapón, mantenga la pluma en la mano. Evite que la pluma entre en contacto con cualquier otro elemento. Esta medida permite garantizar que la pluma no se activa accidentalmente y que la aguja se mantiene limpia.
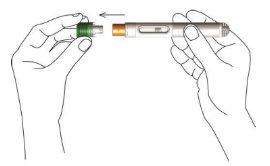
- Forme un pliegue en la piel pellizcando suavemente la piel en el lugar de la inyección con los dedos índice y pulgar. Asegúrese de sujetar el pliegue de piel durante toda la inyección.
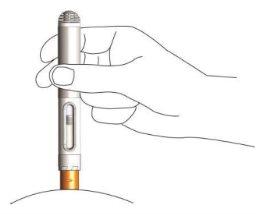
- Desplace la pluma hacia el pliegue de piel (lugar de la inyección) con el protector de la aguja apuntando directamente al lugar de la inyección. Sitúe el protector amarillo de la aguja contra la zona de inyección, de modo que todo el borde del protector de la aguja esté en contacto con la piel.
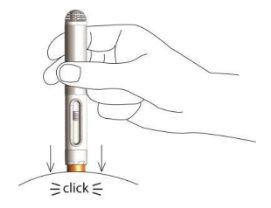
- Aplique presión hacia abajo con la pluma sobre la piel hasta que oiga y perciba un clic.
De este modo, la pluma se activará y la solución se inyectará automáticamente en la piel.
- La inyección dura un máximo de 10 segundos. Notará y oirá un segundo clic una vez que se haya completado la inyección.
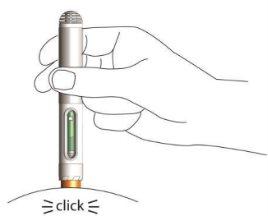
- Espere 2-3 segundos más antes de retirar la pluma de la piel. El protector de seguridad de la pluma se bloquea para evitar lesiones por pinchazos de aguja. Ahora puede soltar el pliegue de piel.
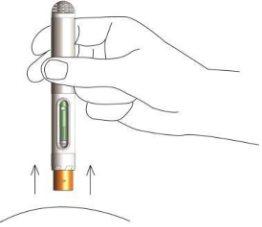
- Inspeccione visualmente la pluma a través de la ventana de visualización. Debería poder ver un plástico verde. Esto significa que se ha inyectado todo el líquido. Deseche la pluma utilizada en el contenedor de objetos punzantes suministrado. Cierre bien la tapa del contenedor y sitúelo fuera del alcance de los niños. En caso de contacto accidental del metotrexato con la piel o los tejidos blandos, enjuague la zona con agua abundante.
Si utiliza más Nordimet del que debe
Siga las recomendaciones de dosis del médico responsable del tratamiento. No cambie la dosis sin consultarlo con su médico.
Si sospecha que ha utilizado más Nordimet del que debe, informe a su médico o póngase inmediatamente en contacto con el hospital más cercano. Acuda a la consulta del médico o al hospital con el envase del medicamento y este prospecto.
Una sobredosis de metotrexato puede producir reacciones tóxicas graves. Los síntomas de sobredosis pueden incluir rápida formación de hematomas o hemorragias, debilidad inusual, llagas en la boca, náuseas, vómitos, heces negras o con sangre, tos con sangre o vómitos con apariencia de posos de café y disminución de la micción (evacuación de la orina de la vejiga). Ver también la sección 4.
Si olvidó usar Nordimet
No use una dosis doble para compensar las dosis olvidadas, pero continúe usando la dosis recetada de forma habitual. Consulte a su médico si tiene dudas.
Si interrumpe el tratamiento con Nordimet
No debe interrumpir o suspender el tratamiento con Nordimet antes de discutirlo con su médico. Si sospecha que está experimentando efectos adversos, consulte inmediatamente a su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Informe a su médico si experimenta un mareo repentino, dificultad para respirar, hinchazón de los párpados, la cara o los labios, erupción cutánea o picor (que afecte especialmente a todo su cuerpo).
Efectos adversos graves
Si desarrolla alguno de los efectos adversos siguientes, póngase inmediatamente en contacto con su médico:
- Inflamación de los pulmones (los síntomas pueden ser enfermedad general, tos seca e irritante, dificultad para respirar, falta de aliento en reposo, dolor en el pecho o fiebre).
- Sangre al escupir o toser.
- Descamación grave o ampollas en la piel.
- Hemorragias (incluida sangre en los vómitos) o formación de hematomas inusuales.
- Diarrea grave.
- Úlceras en la boca.
- Heces negras o alquitranadas.
- Sangre en la orina o las heces.
- Puntitos rojos en la piel.
- Fiebre.
- Coloración amarilla de la piel (ictericia).
- Dolor y dificultad para orinar.
- Sed y/o micción frecuente.
- Ataques (convulsiones).
- Pérdida del conocimiento.
- Pérdida de la visión o visión borrosa.
Se han comunicado los efectos adversos siguientes:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
Pérdida de apetito, náuseas (ganas de vomitar), dolor de estómago, inflamación del revestimiento de la boca, digestión anómala y aumento de las enzimas hepáticas.
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
Menor formación de glóbulos rojos (hematíes) con disminución de los glóbulos blancos (leucocitos) y/o hematíes y/o plaquetas (trombocitos) (leucopenia, anemia, trombocitopenia), dolor de cabeza, fatiga, somnolencia, inflamación de los pulmones (neumonía) con tos seca e improductiva, dificultad para respirar y fiebre, úlceras bucales, diarrea, erupción cutánea, enrojecimiento de la piel y picor.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
Disminución del número de hematíes y trombocitos, inflamación de la garganta, mareos, confusión, depresión, inflamación de los vasos sanguíneos, úlceras y hemorragias en el tracto digestivo, inflamación de los intestinos, vómitos, inflamación del páncreas, trastornos hepáticos, diabetes, disminución de las proteínas sanguíneas, erupción cutánea de tipo herpético, sarpullidos, reacciones similares a las quemaduras solares debido a una mayor sensibilidad de la piel a la luz solar, pérdida de cabello, aumento de los nódulos reumatoides, úlcera cutánea, herpes zóster, dolor articular o muscular, osteoporosis (reducción de la masa ósea), inflamación y úlceras de la vejiga (posiblemente con sangre en la orina), reducción de la función renal, micción dolorosa, inflamación y úlceras en la vagina.
Raros(pueden afectar hasta 1 de cada 1.000 personas)
Infección (incluida la reactivación de una infección crónica inactiva), septicemia, ojos rojos, reacciones alérgicas, shock anafiláctico, disminución del número de anticuerpos en la sangre, inflamación del pericardio, acumulación de líquido en el pericardio, obstrucción del llenado cardíaco debido a la presencia de líquido en el pericardio, trastornos visuales, fluctuaciones del estado de ánimo, baja presión arterial, coágulos sanguíneos, formación de tejido cicatricial en el pulmón (fibrosis pulmonar), neumonía por Pneumocystis jirovecii, interrupción de la respiración, asma, acumulación de líquido en la pleura, inflamación de las encías, hepatitis aguda (inflamación del hígado), oscurecimiento de la piel, acné, puntos rojos o morados debido a hemorragias en los vasos, inflamación alérgica de los vasos sanguíneos, fractura ósea, insuficiencia renal, disminución o ausencia de orina, trastornos electrolíticos, fiebre, cicatrización lenta de heridas.
Muy raros(pueden afectar hasta 1 de cada 10.000 personas)
Reducción de ciertos leucocitos (agranulocitosis), insuficiencia grave de la médula ósea, insuficiencia hepática, inflamación de las glándulas, insomnio, dolor, debilidad muscular, sensación de adormecimiento u hormigueo/menos sensibilidad a la estimulación de lo normal, cambios en el sentido del gusto (sabor metálico), convulsiones, inflamación del revestimiento del cerebro con parálisis o vómitos, alteración de la visión, daños en la retina, vómitos con sangre, megacolon tóxico (agrandamiento del intestino grueso asociado a un dolor intenso), formación defectuosa de esperma (oligospermia), síndrome de Stevens-Johnson, necrólisis epidérmica tóxica (síndrome de Lyell), aumento de la pigmentación de las uñas, pérdida de la libido, problemas para tener una erección, infección alrededor de las uñas, complicaciones graves del tracto digestivo, forúnculos, aumento visible de los pequeños vasos sanguíneos de la piel, trastornos menstruales, secreción vaginal, infertilidad, agrandamiento de las glándulas mamarias en los hombres (ginecomastia) y trastornos linfoproliferativos (crecimiento excesivo de glóbulos blancos).
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles)
Aumento del número de ciertos glóbulos blancos (eosinofilia), ciertos trastornos cerebrales (encefalopatía/leucoencefalopatía), hemorragias nasales, hemorragia pulmonar, daño en los huesos de la mandíbula (secundario a un crecimiento excesivo de glóbulos blancos), proteínas en la orina, sensación de debilidad, destrucción del tejido en el lugar de la inyección, enrojecimiento y descamación de la piel, inflamación.
Solo se observaron reacciones cutáneas locales leves (como sensaciones de quemazón, eritema, hinchazón, decoloración, picazón intensa y dolor) con Nordimet, las cuales disminuyeron durante el tratamiento.
Nordimet puede causar una disminución del número de leucocitos y tal vez disminuya su resistencia a las infecciones. Si sufre una infección con síntomas como fiebre y deterioro grave de su estado de salud general, o fiebre con síntomas de infección local como dolor de garganta/faringe/boca, o problemas urinarios, debe consultar inmediatamente a su médico. Le harán un análisis de sangre para examinar la posible disminución de leucocitos (agranulocitosis). Es importante que informe a su médico si está tomando Nordimet.
Se sabe que el metotrexato causa trastornos óseos como dolor articular y muscular y osteoporosis. Se desconoce la frecuencia de estos riesgos en niños.
Nordimet puede causar efectos adversos graves (en ocasiones, potencialmente mortales). Su médico le realizará pruebas para verificar las anomalías que se desarrollen en la sangre (por ejemplo, bajo recuento de leucocitos, bajo recuento de trombocitos, linfoma) y cambios en los riñones y el hígado.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Nordimet
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta de la pluma precargada y en la caja después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar a una temperatura por debajo de 25 °C.
Conservar la pluma en el embalaje exterior para protegerla de la luz.
No congelar.
No utilice este medicamento si observa que la solución no es transparente o contiene partículas.
Nordimet es de un solo uso. Se debe eliminar cualquier pluma utilizada. Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Nordimet
El principio activo es metotrexato. 1,0 ml de solución contiene 25 mg de metotrexato.
Los demás componentes son cloruro de sodio, hidróxido de sodio y agua para preparaciones inyectables.
Están disponibles las plumas siguientes:
Pluma precargada de 0,3 ml con 7,5 mg de metotrexato
Pluma precargada de 0,4 ml con 10 mg de metotrexato
Pluma precargada de 0,5 ml con 12,5 mg de metotrexato
Pluma precargada de 0,6 ml con 15 mg de metotrexato
Pluma precargada de 0,7 ml con 17,5 mg de metotrexato
Pluma precargada de 0,8 ml con 20 mg de metotrexato
Pluma precargada de 0,9 ml con 22,5 mg de metotrexato
Pluma precargada de 1,0 ml con 25 mg de metotrexato
Aspecto de Nordimet y contenido del envase
Las plumas precargadas con Nordimet contienen una solución inyectable transparente y amarillenta.
Nordimet está disponible en envases que contienen 1 o 4 plumas precargadas y 1 o 4 torundas impregnadas con alcohol, y en envases múltiples que contienen 4 o 6 cajas, conteniendo cada una 1 pluma precargada y una torunda impregnada con alcohol. Nordimet también está disponible en envases múltiples que contienen 3 cajas (con 4 plumas precargadas y torundas de algodón).
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Nordic Group B.V.
Siriusdreef 41
2132 WT Hoofddorp
Países Bajos
Responsable de la fabricación
CENEXI - Laboratoires Thissen
Rue de la Papyrée 2-6
B-1420 Braine-l’Alleud
Bélgica
Sever Pharma Solutions AB
Agneslundsvagen 27
P.O. Box 590
SE-201 25 Malmo
Suecia
FUJIFILM Diosynth Biotechnologies Denmark ApS
Biotek Allé 1
3400 Hillerød
Dinamarca
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento, está disponible en la página web de la Agencia Europea de Medicamentos: http//www.ema.europa.eu.
- País de registro
- Precio medio en farmacia66.94 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a NORDIMET 12,5 MG SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 10 mg/ 1 mlPrincipio activo: MetotrexatoFabricante: Ebewe Pharma Ges.M.B.H. Nfg.KgRequiere recetaForma farmacéutica: INYECTABLE, 15 mgPrincipio activo: MetotrexatoFabricante: Ebewe Pharma Ges.M.B.H. Nfg.KgRequiere recetaForma farmacéutica: INYECTABLE, 20 mgPrincipio activo: MetotrexatoFabricante: Ebewe Pharma Ges.M.B.H. Nfg.KgRequiere receta
Médicos online para NORDIMET 12,5 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de NORDIMET 12,5 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






