
MINIMS TROPICAMIDA 10 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS

Cómo usar MINIMS TROPICAMIDA 10 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el paciente
Minims Tropicamida 10 mg/ml colirio en solución en envase unidosis
tropicamida
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Minims Tropicamida y para qué se utiliza
- Qué necesita saber antes de empezar a usar Minims Tropicamida
- Cómo usar Minims Tropicamida
- Posibles efectos adversos
- Conservación de Minims Tropicamida
- Contenido del envase e información adicional
1. Qué es Minims Tropicamida y para qué se utiliza
Minims Tropicamida en forma de colirio es un medicamento que se aplica de forma tópica en los ojos. Este medicamento contiene el principio activo tropicamida. Se utiliza para agrandar la pupila del ojo y paralizar temporalmente los músculos internos del ojo. Su médico u oftalmólogo puede usarlo para examinarle el ojo o para facilitar procedimientos de cirugía ocular.
Minims Tropicamida se puede utilizar solo o en combinación con otros medicamentos oftálmicos.
Minims Tropicamida está indicado para su uso en adultos (incluidos ancianos), adolescentes y niños mayores de 6 años.
2. Qué necesita saber antes de empezar a usar Minims Tropicamida
No use Minims Tropicamida:
- Si es alérgico a tropicamida o a alguno de los demás componentes de este medicamento (incluidos en la sección 6);
- Si tiene o cree que podría presentar glaucoma. Esto es especialmente importante en los pacientes de edad avanzada.
No utilice este medicamento si cualquiera de los supuestos anteriores es aplicable en su caso. Si no está seguro, consulte a su médico o farmacéutico antes de utilizar Minims Tropicamida.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de usar Minims Tropicamida:
- Si tiene los ojos rojos (síntomas de inflamación);
- Si nota signos de irritación de la piel o reacción cutánea alérgica tras el uso de este medicamento;
- Si presenta elevación de la presión dentro del ojo;
- Si presenta insuficiencia renal o hepática;
- Si no puede utilizar medicamentos denominados alcaloides tropánicos (alcaloides de la belladona), ya que puede ser más probable que experimente efectos secundarios.
Tenga especial cuidado si Minims Tropicamida se administra a niños. Si este medicamento se utiliza incorrectamente durante un tiempo prolongado puede ser absorbido hacia la circulación sistémica, especialmente en los niños. Cierre los párpados y presione suavemente en el ángulo interno del párpado durante 2 minutos para reducir la absorción sistémica. Esto evitará que la solución vaya hacia la nariz y la garganta. Esto es especialmente recomendable en niños.
Niños y adolescentes
Consulte a su médico sobre el uso de Minims Tropicamida en niños y adolescentes, para que decida si este medicamento es adecuado para usted o para su hijo.
No se recomienda el uso de Minims Tropicamida en niños menores de 6 años.
Otros medicamentos y Minims Tropicamida
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría utilizar cualquier otro medicamento.
Esto es especialmente importante si está tomando medicamentos para el tratamiento de la depresión (antidepresivos), la ansiedad (depresores del sistema nervioso central) y las enfermedades del corazón (hipotensores y medicamentos cardiovasculares).
No se conocen interacciones entre tropicamida usada a nivel ocular y otros medicamentos. No obstante,
Minims Tropicamida podría intensificar el efecto de otros medicamentos de la misma clase (midriáticos y ciclopléjicos).
Si usted utiliza Minims Tropicamida junto a otros productos oftálmicos tópicos, deberá esperar al menos 5 minutos entre la administración de cada medicamento. Los geles y pomadas oftálmicas deben administrarse siempre en último lugar.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Su médico puede decidir prescribir este colirio o recetarle otra alternativa.
Se desconoce si tropicamida se excreta en la leche materna humana tras su uso oftálmico.
No hay datos sobre los efectos de tropicamida sobre la fertilidad en humanos.
Conducción y uso de máquinas
Este medicamento puede producir visión borrosa y sensibilidad a la luz. Esto puede afectar a su percepción de la profundidad y, por lo tanto, tiene una influencia negativa sobre la capacidad para conducir. No conduzca ni utilice máquinas hasta que su visión sea clara.
3. Cómo usar Minims Tropicamida
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Este medicamento no debe utilizarse durante más tiempo del recomendado por su médico. Este medicamento puede utilizarse solo o en combinación con otros medicamentos oftálmicos.
Este medicamento debe utilizarse a nivel local en los ojos. Puede utilizar un espejo, que le ayudará a ver lo que está haciendo.
La dosis normal para adultos (incluidos los de edad avanzada), adolescentes y niños a partir de 6 años de edad es 1 gota, seguida por una segunda gota tras un intervalo de 5 minutos. Puede ser necesario instilar 1 gota más después de 30 minutos.
Instrucciones de uso:
- Lávese las manos.
- Retírese las lentes de contacto (si procede).
- Séquese el ojo cuidadosamente con un pañuelo de papel. Desenrosque y tire del tapón para separarlo del cuerpo de Minims Tropicamida (Figura 1).
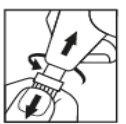
Figura 1
- Tire hacia abajo suavemente de su párpado inferior y luego aplique con cuidado 1 gota dentro del interior del párpado (Figuras 2 y 3). Evite tocar el ojo con el extremo del envase para evitar daños en el ojo y contaminar la solución.


Figura 2 Figura 3
- Cierre los párpados y presione suavemente en el ángulo interno del párpado durante 2 minutos. Esto evitará que la solución vaya hacia la nariz y la garganta (Figura 4).
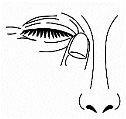 Figura 4
Figura 4
- Repita los pasos 4 y 5 en el otro ojo si fuera necesario; después deseche la unidad, aunque quede algo de solución.
- Lávese las manos después de utilizar el producto.
El colirio es de un solo uso y se proporciona en unidades de un solo uso, para utilizar en ambos ojos si es necesario. Una vez abierto el envase unidosis, se debe utilizar inmediatamente. Desechar cualquier porción no utilizada.
Si usted utiliza Minims Tropicamida junto a otros productos oftálmicos tópicos, deberá esperar al menos 5 minutos entre la administración de cada medicamento. Los geles y pomadas oftálmicas deben administrarse siempre en último lugar.
Uso en niños menores de 6 años
No se recomienda el uso de Minims Tropicamida en niños menores de 6 años.
Si usa más Minims Tropicamida de lo que debe
Es muy poco probable que se produzca una sobredosis.
Los síntomas de sobredosis pueden incluir: enrojecimiento y sequedad de la piel (puede presentarse erupción en niños), visión borrosa, pulso rápido e irregular, fiebre, hinchazón abdominal en lactantes, convulsiones, alucinaciones o pérdida de coordinación.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida. Acuda al hospital, ya que pueden producirse reacciones adversas graves (especialmente en niños).
Si olvidó usar Minims Tropicamida
No debe aplicarse una dosis doble para compensar la dosis olvidada.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Muy frecuentes:pueden afectar a más de 1 de cada 10 pacientes
- Dolor ocular
- Visión borrosa
Frecuencia no conocida:no puede estimarse la frecuencia a partir de los datos disponibles
- Fotofobia (sensibilidad a la luz)
- Picor ocular (transitorio)
- Aumento de la presióndentro del ojo
- Sequedad de boca
- Reacciones alérgicas (hipersensibilidad)
- Náuseas
- Mareo
- Dolor de cabeza
- Desmayo
- Descenso de la presión arterial
Entre los efectos adversos sistémicos de tropicamida se pueden incluir: enrojecimiento de la piel, sequedad de la piel, alteraciones de la frecuencia cardiaca, disminución de la sudoración,disminución de la motilidad gastrointestinal y estreñimiento, incapacidad para vaciar la vejiga totalmente, y disminución de las secreciones nasal, bronquial y lagrimal.
Efectos adversos adicionales en niños
Muy frecuentes:pueden afectar a más de 1 de cada 10 pacientes
- Visión borrosa
Frecuencia no conocida:no puede estimarse la frecuencia a partir de los datos disponibles
- Aumento de la presióndentro del ojo
Con esta clase de fármacos se han notificado, especialmente en niños, cambios en el comportamiento, confusión, excitación, alucinaciones, mayor somnolencia, respiración rápida y cambios en la frecuencia del pulso que requieren asesoramiento y atención médica urgente.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es/. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Minims Tropicamida
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 25 °C. No congelar.
Conservar en el embalaje original para protegerlo de la luz y de la humedad.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja de cartón y en la bolsa del envase, después de «EXP». La fecha de caducidad es el último día del mes que se indica.
No utilice este medicamento si observa cualquier signo visible de deterioro.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto Sigre de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. Pregunte a su farmacéutico cómo deshacerse de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Contenido de Minims Tropicamida
- El principio activo es tropicamida. Un ml del colirio contiene 10 mg de tropicamida.
- Los demás componentes son hidróxido de sodio, ácido clorhídrico y agua purificada.
Aspecto del producto y contenido del envase
Minims Tropicamida es una solución transparente e incolora, estéril y de un solo uso, que prácticamente no contiene partículas visibles y que se presenta en un envase de forma cónica y sellado, provisto de un tapón de rosca. Cada unidad viene envuelta en una bolsa de polipropileno/papel. Cada envase contiene aproximadamente 0,5 ml de solución.
Cada caja contiene 20 envases unidosis estériles de 0,5 ml.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
BAUSCH + LOMB IRELAND LIMITED
3013 Lake Drive
Citywest Business Campus
Dublin 24, D24PPT3
Irlanda
Responsable de la fabricación
Laboratoire Chauvin
Zone Industrielle de Ripotier, 50 Avenue Jean Monnet,
07200, Aubenas, Francia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.
Representante Local en España
Bausch & Lomb S.A.
Avda. Valdelaparra, nº 4, 28108 Alcobendas
Madrid, España.
Tel: 91 – 657 63 00
Este medicamento está autorizado en los Estados miembros del Espacio Económico Europeo con los siguientes nombres:
Hungría:Minims Tropicamide 10 mg/ml oldatos szemcsepp egyadagos tartályban Grecia: Tropicamide/Bausch+Lomb Chipre:Tropicamide/Bausch+Lomb Portugal:Minims Tropicamida 10 mg/mL, colírio, solução em recipiente unidose España:Minims Tropicamida 10 mg/ml colirio en solución en envase unidosis |
Este prospecto ha sido revisado por última vez en Diciembre 2021
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) https://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a MINIMS TROPICAMIDA 10 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSISForma farmacéutica: COLIRIO, 10 mg/mlPrincipio activo: TropicamidaFabricante: Alcon Healthcare S.A.Requiere recetaForma farmacéutica: COLIRIO, -Principio activo: atropineFabricante: Alcon Healthcare S.A.Requiere recetaForma farmacéutica: COLIRIO, 5 mgPrincipio activo: atropineFabricante: Alcon Healthcare S.A.Requiere receta
Médicos online para MINIMS TROPICAMIDA 10 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de MINIMS TROPICAMIDA 10 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















