METALGIAL 500 MG/ML ORAL DROPS IN SOLUTION


How to use METALGIAL 500 MG/ML ORAL DROPS IN SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
Metalgial 500 mg/mloral drops in solution
(metamizole sodium)
Metalgial may cause a lower than normal white blood cell count (agranulocytosis), which can lead to serious and potentially life-threatening infections (see section 4). You should stop taking this medicine and contact your doctor immediately if you experience any of the following symptoms: fever, chills, sore throat, painful sores in your nose, mouth, and throat, or in the genital or anal area. If you have ever had agranulocytosis with metamizole or similar medicines, you should never take this medicine again (see section 2). |
Read the entire leaflet carefully before starting to take this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, ask your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Metalgial and what is it used for
- What you need to know before taking Metalgial
- How to take Metalgial
- Possible side effects
- Storage of Metalgial
- Package contents and additional information
1. What is Metalgial and what is it used for
Metalgial belongs to the group of medicines called "Other Analgesics and Antipyretics".
This medicine is used to treat moderate or severe acute postoperative or post-traumatic pain, colic pain, or pain of tumor origin. It is also used in cases of high fever that does not respond to other measures or other medicines for fever.
Metalgial is available in a 20 ml bottle (with a drop counter) intended for children up to 7 years old (or up to 23 kg in weight), and 30 ml bottles (including a graduated syringe) for use exclusively in children 7 years old and older (or from 23 kg in weight) and adults.
2. What you need to know before taking Metalgial
Do not take Metalgial:
- If you have previously had a significant decrease in a type of white blood cell called granulocytes, which was caused by metamizole or other similar medicines called pyrazolones or pyrazolidines.
- If you have bone marrow problems or have a disorder that affects the way your blood cells are produced or function.
- If you have had previous allergic reactions or hematological reactions (decrease in white blood cells, red blood cells, or platelets in the blood) with medicines containing metamizole or other similar compounds or allergic reactions to any of the other components of this medicine (listed in section 6).
- If you have had symptoms of asthma, rhinitis, or urticaria (red patches or hives on the skin that can cause itching) during treatment with other analgesics such as acetylsalicylic acid, paracetamol, or non-steroidal anti-inflammatory drugs, as you may also be sensitive to metamizole (the active substance of Metalgial).
- If you have acute intermittent porphyria (a disorder of the metabolism of blood pigments that are part of hemoglobin).
- If you have a genetic deficiency of glucose-6-phosphate dehydrogenase.
- If you have had alterations in bone marrow function (blood cell formation); for example, during or after receiving antitumor chemotherapy (antineoplastic) or if you have had diseases related to blood cell formation.
- If you are in the last three months of pregnancy.
- If you have had very severe skin reactions (such as Stevens-Johnson syndrome or toxic epidermal necrolysis) with the use of Metalgial or another medicine that contains metamizole.
Warnings and precautions:
Consult your doctor or pharmacist before starting to take Metalgial.
Low white blood cell count (agranulocytosis).
Metalgial may cause agranulocytosis, a very low level of a type of white blood cell called granulocytes, which are important for fighting infections (see section 4). You should stop taking metamizole and contact a doctor immediately if you experience the following symptoms: chills, fever, sore throat, and painful sores in the mucous membranes (wet surfaces of the body) especially in the mouth, nose, and throat or in the genital or anal area.These disorders can be due to a decrease in the number of white blood cells in the blood (agranulocytosis), platelets (thrombocytopenia), or a failure in the production of all blood cells (aplastic anemia).Your doctor will perform laboratory tests to check the level of your blood cells.
If you take metamizole for fever, some symptoms of agranulocytosis may go unnoticed. Similarly, symptoms may be masked if you are taking antibiotics.
Agranulocytosis can occur at any time during the use of Metalgial and even shortly after stopping metamizole.
You may develop agranulocytosis even if you have used metamizole without problems in the past.
Be especially careful:
- If you experience general discomfort, infection, persistent fever, sore throat, inflammation in the mouth, nose, or throat, lesions in the mouth or genitals, bruising, bleeding, or paleness, you should stop treatment and consult your doctor immediately. These disorders can be due to a decrease in the number of white blood cells in the blood (agranulocytosis), platelets (thrombocytopenia), or a failure in the production of all blood cells (aplastic anemia).
- If you experience dizziness, difficulty breathing, rhinitis, facial swelling, decreased blood pressure, sudden onset of red patches on the skin, stop treatment and consult your doctor. These symptoms may be due to a severe allergic reaction called anaphylactic shock. This reaction is more likely if you have asthma or allergic disorders (atopy).
- If you have bronchial asthma (especially in the presence of nasal mucosa inflammation and nasal polyps), chronic urticaria, or if you are intolerant to dyes and/or preservatives or alcohol, as the risk of severe allergic reactions is higher.
- If you have low blood pressure or hypovolemia (decreased circulating blood volume or any other body fluid), dehydration, or unstable circulation, as the risk of a sudden drop in blood pressure is higher.
- If a skin rash appears that progresses to blistering or lesions in the mucous membranes, you should interrupt treatment and consult a doctor, as severe skin reactions, such as Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug reaction with eosinophilia and systemic symptoms (DRESS), have been reported in association with metamizole treatment. Stop taking metamizole and seek medical attention immediately if you observe any of the symptoms related to these severe skin reactions described in section 4.
- If you have ever had severe skin reactions, you should not resume treatment with Metalgial at any time (see section 4).
- If you have reduced kidney or liver function, as you may have difficulty eliminating the medicine.
- If you are an elderly patient, be especially alert to the appearance of any of the disorders described above, as they may occur more frequently.
- Liver problems: liver inflammation has been reported in patients taking metamizole with symptoms developing within a few days to a few months after starting treatment.
Stop using Metalgial and contact a doctor if you experience symptoms of liver problems, such as discomfort (nausea or vomiting), fever, feeling tired, loss of appetite, dark urine, light-colored stools, yellowing of the skin or the white part of the eyes, itching, rash, or upper abdominal pain. Your doctor will check your liver function.
Do not take Metalgial if you have previously taken a medicine containing metamizole and had liver problems.
Taking Metalgial with food and drinks:
Together with alcohol, the effects of both can be enhanced.
Pregnancy and breastfeeding
Pregnancy
Available data on the use of metamizole during the first three months of pregnancy are limited, but they do not indicate harmful effects on the embryo. In selected cases when there are no other treatment options, single doses of metamizole during the first and second trimester may be acceptable after consulting your doctor or pharmacist and carefully evaluating the benefits and risks of using metamizole. However, in general, the use of metamizole is not recommended during the first and second trimester.
During the last trimester of pregnancy, you should not take Metalgial due to the increased risk of complications for the mother and the baby (bleeding, premature closure of a vital vessel for the fetus, called the ductus arteriosus, which closes naturally after birth).
Breastfeeding
Metamizole degradation products are excreted in breast milk in significant amounts, and it cannot be excluded that there is a risk for the breastfed infant. Therefore, repeated use of metamizole during breastfeeding should be avoided. If a single dose of metamizole is administered, mothers are recommended to express and discard breast milk for 48 hours after administration.
Driving and using machines:
Although no adverse effects on concentration and reaction capacity are expected, at the highest recommended doses, it should be taken into account that these capacities may be affected, and you should avoid using machines, driving vehicles, or engaging in other hazardous activities.
Using Metalgial and other medicines:
Inform your doctor or pharmacist if you are using or have used or may need to use any other medicine.
If you are given cyclosporin (a medicine that prevents transplant rejection) together with Metalgial, it may reduce cyclosporin blood levels, and these should be measured regularly.
If you are given chlorpromazine (a medicine for the treatment of psychoses) together with Metalgial, it may cause a drop in body temperature.
If you are given methotrexate or other anticancer medicines (antineoplastics) together with Metalgial, it may enhance the toxic effects on the blood of the anticancer medicines, especially in elderly patients.
If you are given acetylsalicylic acid together with Metalgial, it may reduce the effect of acetylsalicylic acid on reducing platelet aggregation (antiplatelet) and should be used with caution in patients taking it to protect the heart (cardioprotector).
If you are given the following medicines together with Metalgial, it may decrease the blood levels of these medicines with a possible decrease in clinical efficacy, so they should be used with caution:
- Bupropion, a medicine used to treat depression and/or help quit smoking.
- Efavirenz, a medicine used to treat HIV/AIDS.
- Methadone, a medicine used to treat opioid dependence.
- Valproate, a medicine used to treat epilepsy or bipolar disorder.
- Tacrolimus, a medicine used to prevent organ rejection in transplant patients.
- Sertraline, a medicine used to treat depression.
Metamizole may modify the effect of antihypertensive medicines (medicines that lower blood pressure) and diuretics (medicines that increase fluid elimination).
Important information about some of the components of Metalgial:
This medicine contains 35 mg (1.5 mmol) of sodium per ml (20 drops). This is equivalent to 1.8% of the maximum recommended daily dose of sodium for an adult.
3. How to take Metalgial
Follow the administration instructions for this medication exactly as indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
This medication is for short-term use. Your doctor will indicate the duration of your treatment.
If you estimate that the effect of Metalgial is too strong or too weak, inform your doctor or pharmacist.
This medication should be administered orally.
The dose is established based on the intensity of the pain or fever and the sensitivity of each person to treatment with metamizol. The lowest dose necessary to control the pain and fever should always be selected. Your doctor will indicate how you should take this medication.
The following table shows the recommended single doses and maximum daily doses according to weight or age:
Age (Body weight) | Single dose (Dose per intake) | Maximum daily dose | |||||
Drops | mg | ml | Drops | mg | ml | ||
< 12 months (< 9 kg) | 1-5 | 25-125 | -- | 4-20 | 100–500 | -- |
|
1-3 years (9-15 kg) | 3-10 | 75-250 | -- | 12-40 | 300–1.000 | -- | |
4-6 years (16-23 kg) | 5-15 | 125-375 | -- | 20-60 | 500–1.500 | -- | |
7-9 years (24-30 kg) | -- | 200-500 | 0,4-1,0 | -- | 800–2.000 | 1,6-4,0 |
|
10-12 years (31-45 kg) | -- | 250-750 | 0,5-1,5 | -- | 1.000–3.000 | 2,0-6,0 | |
13-14 years (46-53 kg) | -- | 375-875 | 0,75-1,75 | -- | 1.500–3.500 | 3,0-7,0 | |
≥ 15 years (> 53 kg) | -- | 500–1.000 | 1,0–2,0 | -- | 2.000–4.000 | 4,0-8,0 |
Single doses (dose per intake) may be administered up to four times a day, depending on the maximum daily dose.
The effect of the medication usually appears between 30 and 60 minutes after oral administration.
Use in children and adolescents
For the treatment of pain, children and adolescents up to 14 years of age may take 8 to 16 mg of metamizol per kilogram of body weight in a single dose (see previous table).
In case of fever, a dose of 10 mg of metamizol per kilogram of body weight is generally sufficient in children:
Body weight | Age | Single dose (Dose per intake) | |||
Drops | mg | ml | |||
< 9 kg | < 12 months | 1-3 | 25-75 | -- |
|
9-15 kg | 1-3 years | 4-6 | 100-150 | -- | |
16-23 kg | 4-6 years | 6-9 | 150-225 | -- | |
24-30 kg | 7-9 years | -- | 250-300 | 0,5-0,6 |
|
31-45 kg | 10-12 years | -- | 325-450 | 0,65-0,9 | |
46-53 kg | 13-14 years | -- | 450-525 | 0,9-1,05 |
There are two formats, 20 and 30 ml, which contain a drop counter and an oral syringe, respectively:
20 ml format
In children under 7 years or up to 23 kg in weight, the oral solution should be dosed with the drop counter included in the 20 ml package, which allows dosing in drops (1 drop = 25 mg of sodium metamizol).
30 ml format
In children from 7 years or 23 kg in weight, the oral solution should be dosed using the graduated oral syringe included in the 30 ml package, which allows dosing up to 2 ml (1 ml = 500 mg of sodium metamizol).
Elderly people and patients with poor general health/renal insufficiency
The dose should be reduced in elderly people, in debilitated patients, and in those with decreased renal function, as the elimination of metamizol degradation products may be delayed.
Patients with renal or hepatic insufficiency
Since the elimination rate decreases in cases of renal or hepatic insufficiency, the administration of high repeated doses should be avoided. Only in short-term treatments is a dose reduction not necessary. There is no experience with prolonged treatments.
Instructions for the correct administration of the preparation:
The drops should be administered orally with a little water.
20 ml bottle with drop counter:
- To remove the safety capfrom the bottle, press on the surface of the same (A) and at the same time unscrew in the opposite direction to the clock hands (B).
- Once the cap is removed, place the bottle in a vertical position and completely inverted. DO NOT SHAKE.WAIT A FEW SECONDS UNTIL THE FIRST DROP COMES OUT.
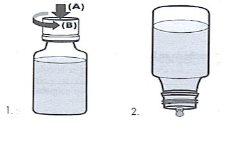
- Close the bottle well after each administration.
Warnings for correct dosing:
Do not extract the contents with a syringe.
Do not use a syringe for dosing.
30 ml bottle with oral syringe:
- To remove the safety capfrom the bottle, press on the surface of the same (A) and at the same time unscrew in the opposite direction to the clock hands (B). Same as in the drawing 1 of the 20 ml format.
- Insert the syringe, pressing on the perforated cap hole.
- Invert the bottle and remove the necessary dose.
- Administer directly or dilute with water.
- The syringe should be washed with water after each intake.
- Close the bottle well after each administration.
Warnings for correct dosing:
Use the syringe contained in this format for correct dosing.
The oral syringe should not be used to dose in drops.
Do not use the bottle to dose in drops.
If you take more Metalgial than you should:
Nausea, vomiting, abdominal pain, deterioration of renal function, and in very rare cases dizziness, drowsiness, coma, convulsions, and decreased blood pressure may occur.
After administration of very high doses of metamizol, a red coloration of the urine may occur, which disappears when treatment is discontinued.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, or the Toxicology Information Service, phone: 91 562 04 20.
If you forget to take Metalgial:
Do not take a double dose to make up for forgotten doses.
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible adverse effects
Like all medications, this medication can cause adverse effects, although not all people experience them.
Mild allergic reactions (e.g., skin and mucous membrane reactions such as itching, burning, redness, swelling) as well as difficulty breathing and gastrointestinal discomfort can progress to more severe forms, e.g., generalized urticaria, swelling of feet, hands, lips, throat, and respiratory tract (angioedema), severe bronchospasm (narrowing of the bronchial walls), heart rhythm disturbances, and decreased blood pressure (sometimes preceded by an increase in blood pressure).
Stop using Metalgial and contact a doctor immediately if you experience any of the following symptoms:
Feeling of illness (nausea or vomiting), fever, feeling of fatigue, loss of appetite, dark urine, light-colored stools, yellowish discoloration of the skin or the white part of the eyes, itching, rash, or pain in the upper abdomen area. These symptoms may be signs of liver damage. See also section 2. Warnings and precautions.
Other adverse effects that may occur with the following frequencies are:
Frequent (may affect up to 1 in 10 people):
- hypotension (decreased blood pressure).
Uncommon (may affect up to 1 in 100 people):
- skin eruptions and reactions.
Rare (may affect up to 1 in 1,000 people):
- allergic reactions that usually occur during or immediately after administration but also hours later,
- skin eruptions and hives,
- decrease in the number of white blood cells in the blood (leukopenia),
- asthma.
Very rare (may affect up to 1 in 10,000 people):
- skin reactions in which blisters or bubbles appear (toxic epidermal necrolysis, Stevens-Johnson syndrome),
- kidney problems with decreased or suppressed urine output,
- increase in the amount of protein excreted in the urine,
- kidney inflammation (interstitial nephritis),
- severe decrease in white blood cells (agranulocytosis) that can cause death due to severe infections,
- decrease in the number of platelets in the blood (thrombocytopenia), in this case, inflammatory lesions in mucous membranes, sore throat, and fever may occur,
- shock (drastic drop in blood pressure).
Frequency not known (cannot be estimated from available data):
- sepsis (severe infection that involves an inflammatory reaction of the entire body and can cause death),
- aplastic anemia (failure of the bone marrow and blood cell production),
- pancytopenia (low number of red, white, and platelet cells simultaneously),
- anaphylactic shock (severe allergic reaction that can cause death),
- Kounis syndrome (a type of cardiac disorder),
- gastrointestinal bleeding,
- abnormal urine coloration.
- liver inflammation, yellowish discoloration of the skin and the white part of the eyes, increased blood levels of liver enzymes.
- severe skin reactions: stop taking metamizol and seek immediate medical attention if you observe any of the following serious adverse effects:
- Red patches that are not raised, or circular or target-shaped patches on the chest, often with central blisters, skin peeling, ulcers in the mouth, throat, nose, genitals, and eyes. These severe skin eruptions may be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome, toxic epidermal necrolysis).
- Generalized redness, elevated body temperature, and enlarged lymph nodes (DRESS or drug hypersensitivity syndrome).
Reporting of adverse effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Conservation of Metalgial
Keep this medication out of sight and reach of children.
No special storage conditions are required.
Do not use Metalgial after the expiration date that appears on the packaging after CAD. The expiration date is the last day of the month indicated.
Medications should not be thrown away through the sewers or in the trash. Deposit the packaging and medications you no longer need at the SIGRE point of the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition of Metalgial
The active ingredient is sodium metamizol. Each ml (20 drops) of solution contains 500 mg of sodium metamizol, equivalent to 467 mg of metamizol.
The other components are: disodium phosphate dodecahydrate, disodium phosphate dihydrate, purified water, raspberry flavor, and sodium saccharin.
Appearance of the product and package contents
Metalgial 500 mg/ml is presented in the form of an oral solution.
There are two types of packages:
20 ml format with drop counter
Brown glass bottle with incorporated drop counter and white polypropylene safety cap for children.
30 ml format with oral syringe
Brown glass bottle with stopper and white polypropylene safety cap for children, plus 2 ml oral syringe with 0.2 ml divisions and 0.1 ml subdivisions.
Marketing authorization holder and manufacturer
Marketing authorization holder:
LABORATORIOS ERN, S.A.
Perú, 228 - 08020 Barcelona, Spain
Manufacturer:
ABC Farmaceutici S.p.A.
Canton Moretti 29 – Loc. S. Bernardo
10090 Ivrea (TO) – Italy
or
LABORATORIOS ERN, S.A.
Gorgs Lladó, 188
08210 Barberá del Vallés, Barcelona, Spain
Date of the last revision of this prospectus: November 2024
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Average pharmacy price2.5 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to METALGIAL 500 MG/ML ORAL DROPS IN SOLUTIONDosage form: CAPSULE, 575 mgActive substance: metamizole sodiumManufacturer: Ababor Pharmaceuticals S.L.Prescription requiredDosage form: CAPSULE, 575 mgActive substance: metamizole sodiumManufacturer: Industria Quimica Y Farmaceutica Vir S.A.Prescription requiredDosage form: CAPSULE, 575 mgActive substance: metamizole sodiumManufacturer: Laboratorios Alter S.A.Prescription required
Online doctors for METALGIAL 500 MG/ML ORAL DROPS IN SOLUTION
Discuss questions about METALGIAL 500 MG/ML ORAL DROPS IN SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions