LEVOSERT ONE 0,02MG CADA 24 HORAS SISTEMA DE LIBERACION INTRAUTERINO


Cómo usar LEVOSERT ONE 0,02MG CADA 24 HORAS SISTEMA DE LIBERACION INTRAUTERINO
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para la usuaria
Levosert One0,02 mg cada 24 horas sistema de liberación intrauterino
levonorgestrel
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento,porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Levosert One y para qué se utiliza
- Qué necesita saber antes de empezar a usar Levosert One
- Cómo usar Levosert One
- Posibles efectos adversos
5 Conservación de Levosert One
- Contenido del envase e información adicional
1. Qué es Levosert One y para qué se utiliza
Ese medicamento es un sistema de liberación intrauterino (SLI) para su inserción en el útero, donde libera lentamente la hormona levonorgestrel.
Se usa para:
Anticoncepción
Este medicamento es un método anticonceptivo eficaz, de larga duración no permanente (reversible).
Este medicamento evita el embarazo al adelgazar el revestimiento de la matriz (útero), haciendo más espeso el moco normal de la abertura del útero (canal cervical), de modo que los espermatozoides no puedan atravesarlo para fecundar el óvulo, y evitando la liberación de los óvulos (ovulación) en algunas mujeres. Además, la presencia del cuerpo en forma de T provoca efectos locales en el revestimiento del útero.
El sistema debe retirarse después de 8 años de uso cuando se utiliza como anticonceptivo.
Tratamiento del sangrado menstrual abundante
Este medicamento también es útil para reducir el flujo sanguíneo menstrual, de modo que lo puede usar si sufre sangrado menstrual (periodos) abundante. Esto se denomina menorragia. La hormona en este medicamento actúa adelagazando el revestimiento de su útero para que haya menos sangrado cada mes.
El sistema deberá retirarse o sustituirse trascurridos 8 años de uso, o antes si reaparece el sangrado menstrual abundante o molesto.
Niños y adolescentes
Este medicamento no está indicado para su uso antes del primer sangrado menstrual (menarquia).
2. Qué necesita saber antes de empezar a usar Levosert One
No use Levosert One
- si está embarazada o sospecha que puede estar embarazada;
- si tiene o ha tenido una enfermedad inflamatoria pélvica;
- Si tiene un flujo vaginal inusual o desagradable, o picor vaginal, ya que esto puede indicar una infección;
- si tiene o ha tenido inflamación del revestimiento del útero tras un parto;
- tiene o ha tenido infección en el útero tras un parto o tras un aborto en los últimos 3 meses;
- si tiene o ha tenido inflamación del cérvix (el cuello del útero);
- si tiene o ha tenido un test de Papanicolau anómalo (cambios en el cervix);
- si tiene o ha tenido problemas en el hígado;
- si tiene un tumor en el hígado;
- si tiene una anomalía uterina, incluyendo miomas uterinos, especialmente aquellos que distorsionan la cavidad uterina;
- si tiene un patrón de sangrado vaginal anómalo;
- si tiene una afección que le hace susceptible de infecciones. Un médico le habrá dicho si tiene este tipo de condición;
- si tiene o ha tenido cáncer dependiente de hormonas, como el cáncer de mama;
- si tiene o ha tenido o sospecha de la existencia de cualquier tipo de cáncer, incluido cancer de la sangre (leucemia), uterino y cervical, a menos que esté en remisión;
- si tiene o ha tenido enfermedad trofoblástica. Un médico le habrá dicho si tiene este tipo de enfermedad;
- si es alérgica a levonorgestrel o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Antes de que se le coloque este medicamento, su médico o enfermero le harán algunas pruebas para asegurarse de que este medicamento es adecuado para usted. Esto incluirá un examen pélvico y también puede incluir otros exámenes, como un examen de los senos, si su médico o enfermero lo consideran apropiado.
Las infecciones genitales tendrán que ser tratadas con éxito antes de que se le pueda colocar este medicamento.
Si tiene epilepsia, comuníqueselo al médico o al enfermero antes de que le coloque este medicamento porque, aunque es poco frecuente, puede producirse un ataque durante la inserción. Algunas mujeres pueden sentir que se desmayan después del procedimiento. Esto es normal y su médico o enfermero le dirá que descanse un rato.
Este medicamento puede no ser adecuado para todas las mujeres.
Este medicamento, al igual que otros anticonceptivos hormonales, no protege contra la infección del VIH (SIDA) ni contra ninguna otra enfermedad de transmisión sexual (p. ej., clamidia, herpes genital, verrugas genitales, gonorrea, hepatitis B y sífilis). Necesitará preservativos para protegerse de estas enfermedades.
Hable con su médico antes de usar Levosert One:
- si tiene o desarrolla migraña, mareos, visión borrosa, dolores de cabeza peores de lo normal, o si tiene dolor de cabeza con más frecuencia que antes;
- si tiene una coloración amarilla en la piel o en el blanco de los ojos (ictericia);
- si es diabética (nivel demasiado alto de azúcar en sangre), tiene tensión arterial alta o niveles anómalos de lípidos sanguíneos;
- si ha tenido cancer que afecta a la sangre (incluida leucemia) que está ahora en remisión
- está bajo tratamiento con esteriodes a largo plazo;
- si ha tenido alguna vez un embarazo ectópico (desarrollo del feto fuera del útero) o antecedentes de quistes ováricos;
- si ha tenido o tiene una enfermedad arterial grave, como ataque al corazón o accidente cerebrovascular;
- si tiene antecedentes de coágulos sanguíneos (trombosis);
- si está tomando otros medicamentos, ya que ciertos medicamentos pueden evitar que este medicamento funcione correctamente;
- si tiene sangrados irregulares;
- si tiene ataques (epilepsia).
Si tiene o ha tenido alguna de las afecciones indicadas anteriormente, su médico decidirá si puede usar este medicamento.
También debe informar a su médico si alguna de estas afecciones se presenta por primera vez mientras tiene colocado este medicamento.
Debe ver a un médico o enfermero lo antes posible si presenta una hinchazón dolorosa en la pierna, dolor repentino en el pecho, o dificultad para respirar, puesto que estos pueden ser signos de un coágulo sanguíneo. Es importante que todo coágulo sanguíneo se trate de inmediato.
Expulsión
Las contracciones musculares del útero durante la menstruación pueden a veces empujar el SLI fuera de su sitio o expulsarlo. Es más probable que esto ocurra si tiene sobrepeso en el momento de la inserción del SLI o si tiene antecedentes de menstruaciones abundantes. Si el SLI se sale de su sitio, es posible que no funcione como es debido y, por tanto, el riesgo de embarazo aumenta. Si el SLI se expulsa, ya no está protegida frente al embarazo.
Los síntomas posibles de una expulsión son dolor y sangrado anormal, pero Levosert One también puede ser expulsado sin darse cuenta. Debido a que Levosert One reduce el flujo menstrual, un aumento del mismo puede ser indicativo de una expulsión.
Es recomendable que verifique los hilos con su dedo, por ejemplo, mientras se ducha. Vea también la sección 3 “Cómo usar Levosert One - ¿Cómo puedo saber si Levosert One está bien colocado?”. Si presentase signos que indiquen la expulsión o no fuera capaz de palpar los hilos, debería usar un método anticonceptivo adicional (como preservativos), y consultar con su profesional sanitario.
Trastornos psiquiátricos:
Algunas mujeres que utilizan anticonceptivos hormonales como este medicamento han notificado depresión o un estado de ánimo deprimido. La depresión puede ser grave y a veces puede inducir pensamientos suicidas. Si experimenta alteraciones del estado de ánimo y síntomas depresivos, póngase en contacto con su médico para obtener asesoramiento médico adicional lo antes posible.
Este medicamento y el hábito tabáquico
Se aconseja a las mujeres que dejen de fumar. Fumar incrementa el riesgo de desarrollar infarto, accidente cerebrovascular, o coágulos sanguíneos.
Uso de tampones y copas menstruales
Se recomienda el uso de compresas. Si se emplean tampones o copas menstruales, debe cambiarlos con cuidado para no tirar de los hilos de extracción de Levosert One.
Otros medicamentos y Levosert One
El efecto de los anticonceptivos hormonales como este medicamento puede verse reducido por medicamentos que aumentan la cantidad de enzimas producidas por el hígado. Informe a su médico si está tomando:
- fenobarbital, fenitoína o carbamazepina (para tratar la epilepsia);
- griseofulvina (un antifúngico);
- rifampicina o rifabutina (antibióticos);
- nevirapina o efavirenz (para el VIH).
Informe a su médico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento. Este medicamento no debe usarse simultáneamente con otro anticonceptivo hormonal.
Embarazo, lactancia y fertilidad
No debe usar este medicamento durante el embarazo o si sospecha que puede estar embarazada.
¿Me puedo quedar embarazada mientras uso este medicamento?
Es muy raro que una mujer se quede embarazada teniendo colocado este medicamento.
No tener un periodo no necesariamente significa que está embarazada. Algunas mujeres pueden no tener reglas mientras usan el sistema.
Si no ha tenido un periodo durante 6 semanas, considere hacerse una prueba de embarazo. Si es negativa, no hay necesidad de realizarse más pruebas, a menos que tenga otros síntomas de embarazo, como por ejemplo náuseas, cansancio o sensibilidad en los pechos.
Si se queda embarazada con el dispositivo colocado, contacte con su médico lo antes posible para descartar un embarazo ectópico (desarrollo del feto fuera del útero) y se le pueda extraer este medicamento para reducir el riesgo de un aborto espontáneo. Sin embargo, si Levosert One se deja colocado durante el embarazo, no sólo aumenta el riesgo de sufrir un aborto espontáneo, sino también el riesgo de parto prematuro. Si Levosert One no se puede extraer, hable con su profesional sanitario sobre los beneficios y riesgos de continuar el embarazo. Si el embarazo continúa, será monitorizada de cerca durante su embarazo y debe contactar con su médico de inmediato si experimenta calambres estomacales, dolor de estómago o fiebre.
Levosert One contiene una hormona denominada levonorgestrel, y ha habido casos aislados de efectos genitales en los bebés si se exponen a dispositivos intrauterinos de levonorgestrel mientras están en el útero.
¿Y si quiero tener un bebé?
Si desea tener un bebé, pídale a su médico que le extraiga este medicamento. Su nivel normal de fertilidad volverá muy rápidamente una vez el sistema se haya extraído.
¿Puedo dar el pecho mientras uso este medicamento?
En la leche materna se encuentran cantidades muy pequeñas de la hormona de este medicamento. No se espera que haya riesgo alguno para el recién nacido. Puede continuar la lactancia durante el uso de este medicamento.
Conducción y uso de máquinas
No hay efectos conocidos sobre la capacidad de conducir y utilizar máquinas.
Levosert One contiene sulfato de bario
La estructura en T de este medicamento contiene sulfato de bario, que lo hace visible con rayos X.
3. Cómo usar Levosert One
Solamente un médico o enfermero con formación específica puede colocar el sistema (ver las instrucciones especiales para la inserción en el envase).
El profesional le explicará el procedimiento de colocación y cualquier riesgo asociado a su uso. Después será examinada por su médico o enfermero antes de la inserción de este medicamento. Si tiene cualquier duda sobre su uso puede consultarlo con ellos.
Empezar a usar Levosert One
- Antes de insertar Levosert One, es necesario asegurarse de que no está embarazada.
- Debería tener Levosert One insertado en un plazo de 7 días a partir del inicio de la menstruación. Cuando Levosert One se inserta en estos días, Levosert One actúa de inmediato y evitará que quedes embarazada.
- Si no puede tener Levosert One insertado en un plazo de 7 días a partir del inicio de la menstruación o si su periodo menstrual llega en momentos impredecibles, Levosert One puede ser insertado en cualquier otro día. En este caso, no debe haber tenido relaciones sexuales sin anticoncepción desde su último periodo menstrual, y debe tener una prueba de embarazo negativa antes de la inserción. Además, Levosert One puede no prevenir el embarazo de manera confiable de inmediato. Por lo tanto, debe usar un método de barrera anticonceptiva (como preservativos) o abstenerse de relaciones sexuales vaginales durante los primeros 7 días después de la inserción de Levosert One.
- Levosert One no es adecuado para uso como anticonceptivo de emergencia (anticonceptivo postcoital).
Empezar a usar Levosert One después del parto
- Levosert One puede ser insertado después del parto una vez que el útero haya vuelto a su tamaño normal, pero no antes de las 6 semanas posteriores al parto (ver sección 4 “Posibles efectos adversos – Perforación”).
- Ver también “Empezar a usar Levosert One” más arriba para conocer qué más necesita saber sobre el momento de la inserción.
Empezar a usar Levosert One después de un aborto
Levosert One puede ser insertado inmediatamente después de un aborto, si el embarazo fue de menos de 3 meses de duración siempre que no haya infecciones genitales. Entonces, Levosert One funcionará de inmediato.
Reemplazo de Levosert One
Levosert One puede ser reemplazado por un nuevo Levosert One en cualquier momento de su ciclo menstrual. Entonces, Levosert One funcionará de inmediato.
Cambio de otro método anticonceptivo (como anticonceptivos hormonales combinados, implante)
- Levosert One puede puede ser insertado inmediatamente si hay una certeza razonable de que no está embarazada.
- Si han pasado más de 7 días desde el inicio de su sangrado menstrual, debe abstenerse de mantener relaciones sexuales vaginales o usar protección anticonceptiva adicional durante los próximos 7 días.
Colocación de Levosert One
El examen realizado por su profesional sanitario antes de la colocación puede incluir:
- una prueba de citología cervical (frotis de Papanicolaou);
- un examen de las mamas;
- otras pruebas, por ejemplo para infecciones, incluidas enfermedades de transmisión sexual, prueba de embarazo, según sea necesario. Su profesional sanitario también hará un examen ginecológico para determinar la posición y el tamaño del útero.
Después del examen ginecológico
- Un instrumento denominado espéculo se introduce en la vagina, y el cérvix puede ser limpiado con una solución antiséptica. Entonces, Levosert One es colocado en el útero utilizando un tubo fino y flexible de plástico (el tubo de colocación). Se puede aplicar anestesia local en el cérvix antes de la colocación.
- Algunas mujeres se sienten mareadas o se desmayan durante la colocación o después de que Levosert One sea colocado o retirado.
- Puede experimentar algo de dolor y sangrado durante o justo después de la colocación.
Después de la colocación de Levosert One debería recibir una tarjeta de recordatorio para la paciente de su médico para exámenes de seguimiento. Lleve esta tarjeta con usted a cada cita programada.
¿Con qué rapidez funciona Levosert One?
Anticoncepción
Si Levosert es insertado en su útero ya sea durante su periodo menstrual o dentro de los 7 días posteriores al inicio de su periodo o tiene un dispositivo y es momento de reemplazarlo por uno nuevo o si acaba de tener un aborto, usted está protegida contra el embarazo desde el momento en que le colocan el sistema.
Sangrado menstrual abundante
Este medicamento normalmente logra una reducción significativa de la pérdida de sangre menstrual en el plazo de 3 a 6 meses de tratamiento.
¿Cómo afectará Levosert One a mis periodos?
Muchas mujeres tienen manchados (una pequeña pérdida de sangre) en los primeros 3-6 meses tras la colocación del sistema. Otras tendrán sangrados prolongados o abundantes. Sin embargo, puede presentar un aumento de sangrado, normalmente en los primeros 2 a 3 meses, antes de que se logre una reducción de la pérdida de sangre. En general, tiene más posibilidades de tener menos días de sangrado cada mes y puede que incluso deje de tener el periodo. Esto se debe al efecto de la hormona (levonorgestrel) en el revestimiento del útero. Si no se consigue una reducción notable de la pérdida de sangre al cabo de 3 a 6 meses, deben considerarse otros tratamientos.
Si le han colocado este medicamento hace mucho tiempo y luego empieza a tener problemas de sangrado, póngase en contacto con su médico o profesional sanitario para que le aconseje.
¿Con qué frecuencia debería hacerme un control del sistema?
Debería comprobar su Levosert One al cabo de 4 a 6 semanas después de la colocación, y a partir de entonces regularmente, al menos una vez al año hasta su retirada. Su médico podrá determinar con qué frecuencia y qué tipos de controles son requeridos en su caso particular. Lleve la tarjeta de recordatorio para la paciente que recibió de su médico a cada cita programada. Además, debe ponerse en contacto con su médico si presenta alguno de los síntomas descritos en la sección 2 “Advertencias y precauciones”.
¿Cómo puedo saber si el sistema está en su lugar?
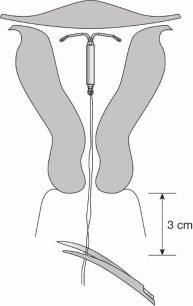
Tras cada periodo menstrual, puede buscar los dos hilos finos que están unidos al extremo inferior del sistema. Su médico le enseñará cómo hacerlo.
No tirede los hilos, porque podría sacarlo accidentalmente. Si no encuentra los hilos, contacte con su médico o enfermero lo antes posible y evite las relaciones sexuales o use un método anticonceptivo de barrera (como los preservativos) mientras tanto. Puede que los hilos simplemente hayan entrado en el útero o canal cervical. Si su médico o enfermero sigue sin encontrar los hilos, puede que se hayan roto, o este medicamento podría haber salido por sí solo, o en raros casos puede haber perforado la pared del útero (perforación uterina, ver sección 4).
También debería ir al médico si puede tocar el extremo inferior del dispositivo mismo, o si usted o su pareja sienten dolor o molestias durante las relaciones sexuales.
Si el sistema se sale completamente o en parte, puede que no esté protegida contra el embarazo. Es raro, pero posible, que esto suceda sin que usted se dé cuenta durante el periodo menstrual. Un incremento inusual de la cantidad de sangrado durante el periodo puede ser un signo de que esto ha sucedido. Informe a su médico o profesional sanitario si presenta cambios inesperados en su patrón de sangrado.
Retirada de Levosert One
Levosert One debe ser retirado o reemplazado después de 8 años de uso, o antes si reaparece el sangrado menstrual abundante o molesto.
Su médico puede retirar el sistema fácilmente en cualquier momento, después de lo cual es posible quedarse embarazada. Algunas mujeres se sienten mareadas o se desmayan durante o después de la retirada de Levosert One. Puede experimentar algo de dolor y sangrado durante la retirada de Levosert One.
Continuación de la anticoncepción después de la retirada
Si no desea quedarse embarazada, Levosert One no debe ser retirado después del séptimo día del ciclo menstrual (periodo mensual) a menos que utilice otros métodos anticonceptivos (por ejemplo, preservativos) durante al menos 7 días antes de la retirada del SLI.
Si tiene periodos (menstruaciones) irregulares o no tiene periodos, debería utilizar un método anticonceptivo de barrera durante 7 días antes de la retirada.
También, un nuevo Levosert One puede ser colocado inmediatamente después de la retirada, en cuyo caso no se necesita protección adicional. Si no desea continuar con el mismo método, pregunte a su médico sobre otros métodos anticonceptivos fiables.
Si tiene más preguntas sobre el uso de este medicamento, pregunte a su médico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Con este medicamento, los efectos adversos son más frecuentes durante los primeros meses tras haber colocado el sistema y van disminuyendo conforme pasa el tiempo.
Si usted experimenta alguno de los siguientes efectos adversos graves, por favor contacte con su médico o enfermero inmediatamente:
- Dolor intenso o fiebre que desarrolla poco tiempo después de la inserciónpuede significar que tiene una infección grave que debería tratarse inmediatamente. En raros casos puede ocurrir una infección muy grave (sepsis).
- Dolor intenso y sangrado continuadoya que esto puede ser un signo de daño o desgarro de la pared del útero (perforación). La perforación es poco frecuente, pero ocurre con mayor frecuencia durante la inserción de este medicamento, aunque puede no detectarse hasta algún tiempo después. Si este medicamento se ha alojado fuera de la cavidad del útero, no es efectivo para prevenir el embarazo y debe ser retirado lo antes posible; en muy raras ocasiones esto puede requerir cirugía. El riesgo de perforación es bajo, pero se incrementa en mujeres en periodo de lactancia o mujeres que han tenido un bebé hasta 36 semanas antes de la inserción y puede que se incremente en mujeres con el útero fijo inclinado hacia atrás (útero en retroversión y fijo). Si sospecha que puede haber sufrido una perforación, busque inmediatamente asistencia médica y recuérdele que tiene insertado este medicamento, especialmente si no fue la persona que se lo insertó..
Posibles signos y síntomas de la perforación pueden incluir:
- dolor intenso (como calambres menstruales) o más dolor del esperado
- sangrado abundante (tras la inserción)
- dolor o sangrado que continua más de unas pocas semanas
- cambios repentinos en los periodos
- dolor durante relaciones sexuales
- si ya no puede sentir los hilos de este medicamento (ver sección 3 “Cómo usar Levosert “¿Cómo puedo saber si el sistema está en su lugar?”).
- Dolor en la parte baja del abdomen especialmente si también tiene fiebre o ha tenido una falta o tiene un sangrado inesperado,ya que esto puede ser un signo de embarazo ectópico (desarrollo del feto fuera del útero). El riesgo absoluto de embarazo ectópico en usuarias de este medicamento es bajo. Sin embargo, cuando una mujer queda embarazada con este medicamento dentro, la probabilidad de embarazo ectópico se incrementa.
- Dolor en la parte baja del abdomen o experimenta relaciones sexuales difíciles o dolorosasya que puede ser un signo de quistes ováricos o enfermedad inflamatoria pélvica. Esto es importante ya que las infecciones pélvicas pueden reducir sus probabilidades de tener un bebé y pueden aumentar el riesgo de embarazo ectópico.
Otros efectos adversos
Muy frecuentes(pueden afectar a más de 1 de cada 10 mujeres) pueden incluir:
- ausencia de menstruaciones, ligeras o infrecuentes (véase "¿Cómo afectará Levosert One a mis menstruaciones?" en la sección 3.
- sangrado vaginal incluyendo manchado.
- infecciones vaginales y de los genitales externos (vulva) causadas por hongos o bacterias;
- granos (acné);
Frecuentes(pueden afectar hasta 1 de cada 10 mujeres) pueden incluir:
- depresión, nerviosismo u otros cambios de humor;
- apetito sexual reducido;
- cefalea;
- migraña;
- sensación de desmayo (presíncope);
- mareos;
- dolor de espalda;
- malestar abdominal;
- malestar (náuseas);
- abdomen hinchado;
- vómitos;
- periodos dolorosos;
- aumento del flujo vaginal;
- pechos sensibles y doloridos;
- espasmo del útero;
- Este medicamento se sale de su lugar;
- aumento de peso.
Poco frecuentes(pueden afectar hasta 1 de cada 100 mujeres) pueden incluir:
- desmayo;
- eczema;
- inflamación del cuello del útero (cervicitis);
- hinchazón o inflamación en piernas o tobillos;
- aumento del crecimiento de vello en la cara y el cuerpo;
- pérdida de cabello;
- picazón en la piel (prurito);
- decoloración de la piel o aumento de pigmentación de la piel, especialmente en la cara (cloasma).
Raros(pueden afectar hasta 1 de cada 1.000 mujeres) pueden incluir:
- erupción cutánea, picores.
Comunicación de efectos adversos
Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaram.es.Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Levosert One
Este medicamento no requiere condiciones especiales de conservación.
Conservar en el embalaje original y mantener el blíster sellado en la caja exterior para protegerlo de la luz.
Mantener el envase perfectamente cerrado. Solo su médico o profesional sanitario debe abrirlo.
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en la caja después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Levosert One
El principio activo es levonorgestrel.
Levosert One contiene 52 mg de levonorgestrel contenido en una sustancia denominada polidimetilsiloxano, rodeada por una membrana también de polidimetilsiloxano.
Aspecto del producto y contenido del envase
- Levosert One está formado por un cuerpo en forma de T hecho de un plástico denominado polietileno. Esta estructura lleva un reservorio de hormona que permite liberarla de forma gradual en el útero.
- Hay dos hilos finos, hechos de polipropileno y azul de ftalocianina de cobre, unidos al extremo inferior de esta estructura. Estos hilos permitirán extraer facilmente el dispositivo y a usted y a su médico comprobar que el dispositivo está en su lugar.
El SLI Levosert One junto con el dispositivo aplicador se presentan acondicionados de forma individual en un blíster de plástico termoformado con cubierta despegable dentro de una caja de cartón. El blíster estéril se envasa dentro de una caja con el prospecto y la tarjeta recordatorio para la paciente.
Tamaños de envase:
1 sistema de liberación intrauterino con dispositivo aplicador.
Multipack con 5 envases con un sistema de liberación intrauterino con un dispositivo aplicador.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Gedeon Richter Plc.
Gyömroi út 19-21.
1103 Budapest
Hungría
Responsable de la fabricación
Odyssea Pharma S.A.
Rue du Travail 16
4460 Grâce Hollogne
Bélgica
Gedeon Richter Plc.
Gyömroi út 19-21
1103 Budapest
Hungría
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Gedeon Richter Ibérica S.A.
Sabino Arana, 28 4º 2ª
08028 Barcelona
España
+34 93 2034300
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria Levosert One
Chipre Levosert One
Alemania Levosert One
Dinamarca Levosert One
España Levosert One 0,02 mg cada 24 horas sistema de liberación intrauterino
Irlanda Levosert SHI
Islandia Levosertone
Italia Benilexa
Malta Levosert One
Noruega Levosert Single-Handed Inserter
Suecia Levosert Single-Handed Inserter
Eslovenia Levosert SHI
Reino Unido Benilexa One Handed
Fecha de la última revisión de esteprospectojunio 2024
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la {Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
_________________________________________________________________________________
Esta información está destinada únicamente a profesionales del sector sanitario:
Instrucciones de uso y manipulación
Lista de comprobación para el médico prescriptor
Hágase las siguientes preguntas antes de prescribir/insertar este medicamento:
- ¿He comprobado que las necesidades de la paciente cumplen con las indicaciones de anticoncepción o sangrado menstrual abundante y con la duración de uso, de hasta ocho años?
- ¿He cumplimentado la tarjeta de la paciente incluida en el envase y se la he dado a la paciente como recordatorio? (toda inserción de más de ocho años de duración debe comunicarse como uso no autorizado)
Lea las siguientes instrucciones de uso atentamente, puesto que puede haber alguna diferencia en el tipo de dispositivo aplicador en comparación con otros DIU que haya usado anteriormente:
Instrucciones de inserción
Debe ser insertado por un profesional sanitario utilizando una técnica aséptica.
Se recomienda que Levosert One solo debería ser insertado por profesionales sanitarios que estén experimentados en la colocación de sistemas de liberación intrauterina (SLI) y/o que hayan recibido formación suficiente sobre el procedimiento de inserción de Levosert One y hayan leído atentamente estas instrucciones antes de la inserción de Levosert One.
Levosert One se suministra en un envase estéril que no debe abrirse hasta que sea necesario para su inserción. No reesterilizar. Para un solo uso. El producto, una vez expuesto, debe manejarse con precauciones asépticas. Si se rompe el sellado del envase estéril, el producto debe desecharse (ver instrucciones de eliminación en la sección 6.6). No utilizar si el envase interno está dañado o abierto. No insertar después de la fecha de caducidad indicada en la caja y en el blíster después de CAD. La fecha de caducidad es el último día del mes que se indica.
Para conocer el momento de la inserción, consulte la sección 4.2 de la ficha técnica.
Levosert One incluye dentro del envase una tarjeta recordatorio para la paciente. Completar la tarjeta recordatorio para la paciente y entregarla a la paciente después de la inserción.
Preparación para la inserción
- Examine a la paciente para descartar contraindicaciones para la inserción de Levosert One (ver secciones 4.3 y 4.4 bajo Exploración médica).
- Coloque un espéculo, visualice el cuello uterino y, a continuación, haga una limpieza minuciosa del cuello uterino y la vagina con una solución antiséptica adecuada.
- El profesional sanitario puede contar con la colaboracion de personal auxiliar si lo considera necesario.
- Sujete el labio anterior del cuello uterino con unas pinzas tenáculo u otras pinzas para estabilizar el útero. Si el útero está en retroversión, puede ser más apropiado sujetar el labio posterior del cuello uterino. Se puede aplicar una ligera tracción con las pinzas para enderezar el canal cervical. Las pinzas deben permanecer en su posición y se debe mantener una tracción suave sobre el cuello uterino durante todo el procedimiento de inserción.
- Introducir una sonda uterina avanzadas a través del canal cervical hasta el fondo para medir la profundidad. Si la profundidad uterina es < 5,5 cm, interrumpir el procedimiento. Confirme la dirección de la cavidad uterina y excluya cualquier evidencia de anomalías intrauterinas (p. ej., septo, miomas submucosos) o un anticonceptivo intrauterino previamente insertado que no haya sido retirado. Si se encuentra dificultad, considere la dilatación del canal cervical. Si se requiere dilatación cervical, se debe considerar el uso de analgésicos y/o un bloqueo paracervical.
Descripción
Figura 1a: Sistema de Liberación Intrauterino (SLI) Levosert One
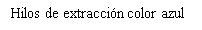

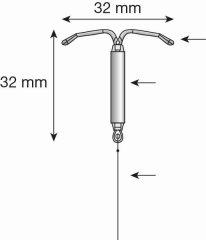
Figura1b:SLI con insertor Levosert One


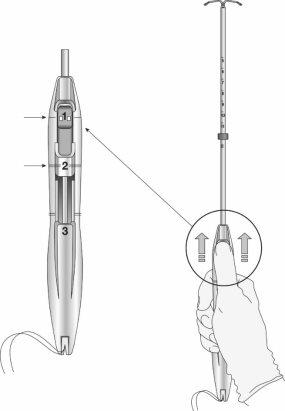
Figura 2: Deslizadores de inserción




Preparación para la inserción
Paso 1: Abrir el envase estéril de Levosert One
- Saque de la caja el blíster sellado que contiene el dispositivo.
- Inspeccione el blíster sellado y no utilice el producto si el embalaje, el insertor o el SLI están dañados.
- Coloque el blíster en una superficie plana con la tapa despegable hacia arriba.
- Retire la tapa despegable.
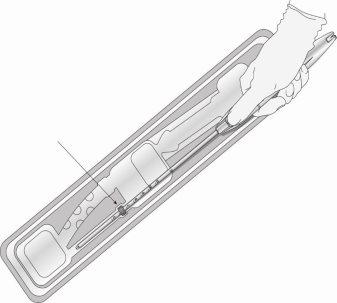
Paso 2: Sacar el insertor del blíster (figura 3)
Figura 3

Paso 3: Deslizar completamente los deslizadores hacia adelante para cargar el SLI (figura 4)
Figura 4




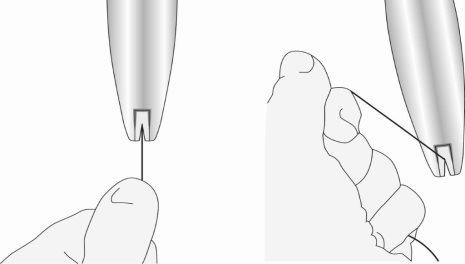
Paso 4: Cargar el SLIen el insertor
- Asegúrese de que los brazos del SLI están horizontales (alineados con el plano horizontal del mango y el marcador); ajuste la rotación del SLI según sea necesario utilizando la superficie plana estéril del blíster.
- Manteniendo la presión hacia delanteen el deslizador azul, tire suavemente de los hilos hacia atráspara cargar el SLI en el tubo de inserción. Asegúrese de que se aplica una tensión uniforme a ambos hilos al tirar. Tire de los hilos hacia arriba o hacia abajo para bloquear los hilosen la hendidura en la base del mango (figura 5); debe bloquear los hilos en la hendidura para evitar que el SIU se salga de la parte superior del tubo de inserción. Una vez que los hilos estén bloqueados en la hendidura, deje de sujetarlos.
Figura 5: Bloqueo de los hilos en la hendidura
- Una vez cargado el SLI, siga manteniendo la presión hacia delante en el deslizador AZUL para mantener la posición correcta del SLI.
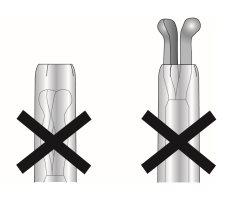
- Cuando está correctamente cargado, el SLI está completamente dentro del tubo de inserción con las puntas de los brazos formando una cúpula semiesférica en la parte superior del tubo (figura 6, imagen 1).
Figura 6: Posición del SLI en el tubo de inserción
Imagen 1


Imagen 2

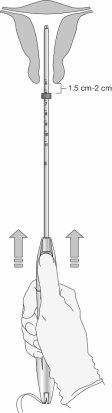
Paso 5: Ajuste del marcador (figura 7)
Figura 7


Paso 6: Inserción del SLI en el útero (figura 8)
Figura 8


Paso 7: Liberar y abrir los brazos del SLI
Figura 9



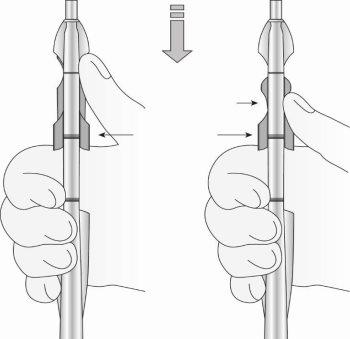
Figura 10: Mover el dispositivo en dirección al fondo uterino

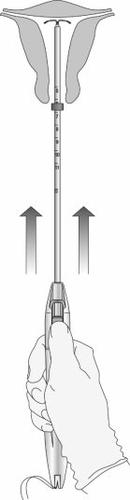
Paso 8: Liberación del SLI y finalización del procedimiento
Figura 11: Liberación del SLI del tubo de inserción


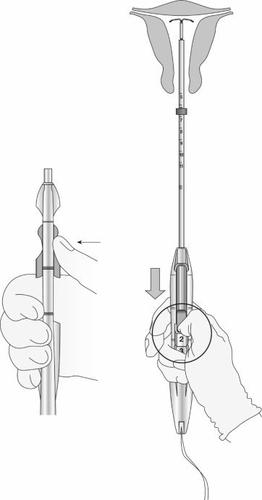
Figura 12: Indicador verde visible e hilos liberados de la hendidura

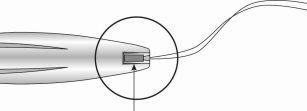
Figura 13: Cortar los hilos a unos 3 cm del cuello del útero
|
La inserción de Levosert One se ha completado.
Información importante a tener en cuenta durante o después de la inserción:
- si sospecha que el SLI no está en la posición correcta:
- Compruebe la inserción con una ecografía u otra prueba radiológica adecuada.
- Si se sospecha que la inserción es incorrecta, retire el SLI. No vuelva a insertar el mismo SLI después de retirarlo.
IMPORTANTE!
En caso de una inserción difícil y/o de dolor o sangrado excepcionales durante o tras la inserción, se debe realizar una exploración física y una ecografía inmediatamente para descartar la perforación del cuerpo o cuello uterino. La exploración física por sí sola (incluida la comprobación de los hilos) puede no ser suficiente para excluir una perforación parcial. Si es necesario, extraiga el sistema e inserte un nuevo sistema estéril.
Después de la inserción, las mujeres deben ser reexaminadas al cabo de 4 a 6 semanas para comprobar los hilos y asegurar que el dispositivo está en la posición correcta. Informe sobre cualquier caso de perforación uterina o dificultades de inserción a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es.
Retirada/sustitución
El SLI se extrae tirando con suavidad de los hilos con un fórceps. El uso de fuerza excesiva o instrumentos afilados durante la extracción puede provocar la rotura del sistema.
Si los hilos no son visibles y se descubre que el sistema está en la cavidad uterina en el examen de ultrasonido, se puede extraer usando unos fórceps estrechos. Esto puede requerir dilatación del canal cervical o intervención quirúrgica.
Después de la extracción del SLI, se debe examinar el sistema para comprobar que está intacto y que se ha retirado por completo. Durante las extracciones difíciles, se han notificado casos aislados en los que el cilindro de la hormona se ha deslizado sobre los brazos horizontales, escondiéndolos totalmente dentro del cilindro. Esta situación no requiere ninguna otra intervención una vez se haya comprobado que el SLI está completo. Las protuberancias de los brazos horizontales normalmente evitan la completa separación del cilindro del cuerpo en forma de T.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a LEVOSERT ONE 0,02MG CADA 24 HORAS SISTEMA DE LIBERACION INTRAUTERINOForma farmacéutica: DISPOSITIVO INTRAUTERINO, 13,5 mg levonorgestrelPrincipio activo: plastic IUD with progestogenFabricante: Bayer Hispania S.L.Requiere recetaForma farmacéutica: DISPOSITIVO INTRAUTERINO, 19,5 mgPrincipio activo: plastic IUD with progestogenFabricante: Bayer Hispania S.L.Requiere recetaForma farmacéutica: DISPOSITIVO INTRAUTERINO, 52 mg/ tasa de liberación inicial de 0,02 mg cada 24 hPrincipio activo: plastic IUD with progestogenFabricante: Gedeon Richter Plc.Requiere receta
Médicos online para LEVOSERT ONE 0,02MG CADA 24 HORAS SISTEMA DE LIBERACION INTRAUTERINO
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de LEVOSERT ONE 0,02MG CADA 24 HORAS SISTEMA DE LIBERACION INTRAUTERINO, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes