
KLISYRI 10 MG/G POMADA

Cómo usar KLISYRI 10 MG/G POMADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Klisyri 10 mg/g pomada
tirbanibulina
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted,y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Klisyri y para qué se utiliza
- Qué necesita saber antes de empezar a usar Klisyri
- Cómo usar Klisyri
- Posibles efectos adversos
- Conservación de Klisyri
- Contenido del envase e información adicional
1. Qué es Klisyri y para qué se utiliza
Klisyri contiene el principio activo tirbanibulina. Se utiliza para el tratamiento de la queratosis actínica leve en adultos. La queratosis actínica consiste en un área áspera de piel que se desarrolla en personas que se han expuesto mucho al sol durante mucho tiempo. Klisyri solo debe usarse para la queratosis actínica plana de la cara y el cuero cabelludo.
2. Qué necesita saber antes de empezar a usar Klisyri
No use Klisyri
- si es alérgico a tirbanibulina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Klisyri
- No use Klisyri hasta que la zona que se va a tratar haya sanado de cualquier medicamento, procedimiento o tratamiento quirúrgico anterior. No se aplique Klisyri en heridas abiertas ni piel lacerada.
- Lávese las manos si toca la zona donde se ha aplicado la pomada.
- No permita que Klisyri entre en contacto con los ojos. En caso de contacto accidental con los ojos, enjuágueselos con agua abundante, solicite atención médica lo antes posible y lleve consigo este prospecto.
- No aplique la pomada en zonas internas del cuerpo, dentro de las fosas nasales, en el interior del oído ni en los labios. En caso de contacto accidental de la pomada con cualquiera de estas zonas, lávesela enjuagando la zona con agua.
- No ingiera este medicamento. En caso de ingestión accidental de este medicamento, beba mucha agua, solicite atención médica y lleve consigo este prospecto.
- Informe a su médico si tiene problemas con su sistema inmune.
- Esté atento a la aparición de nuevas placas rojas y escamosas, llagas abiertas y lesiones verrugosas o con relieve alrededor de la zona de tratamiento. Si observa alguna de ellas, consulte a su médico inmediatamente.
- Después de usar Klisyri, evite la práctica de actividades que puedan provocar un sudor excesivo, así como la exposición a la luz solar en la medida de lo posible (incluyendo lámparas solares y cabinas de bronceado). Cuando esté al aire libre, use ropa protectora y gorra.
- No cubra la zona tratada con vendas después de usar Klisyri.
- No se aplique más pomada de la que le haya recomendado su médico.
- No se aplique la pomada más de una vez al día.
- No permita que las mascotas ni otras personas toquen la zona tratada durante unas 8 horas después de haberse aplicado la pomada. Si se toca la zona tratada, se debe lavar la zona de contacto de la otra persona o la mascota.
- Póngase en contacto con su médico si experimenta reacciones cutáneas a este medicamento en la zona tratada que se agravan (ver sección 4).
Niños y adolescentes
No administre este medicamento a niños y adolescentes menores de 18 años porque no desarrollan la queratosis actínica.
Otros medicamentos y Klisyri
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Si ha usado anteriormente Klisyri o medicamentos similares, informe a su médico antes de comenzar el tratamiento.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Klisyri no debe utilizarse durante el embarazo.
Conducción y uso de máquinas
No se espera que este medicamento afecte a su capacidad para conducir o utilizar máquinas.
Klisyri contiene propilenglicol
El propilenglicol puede provocar irritación en la piel.
3. Cómo usar Klisyri
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Este medicamento está indicado para tratar un área máxima de 25 cm2 en un único ciclo de tratamiento de cinco días. Si la zona tratada no muestra un aclaramiento completo aproximadamente a las 8 semanas de haber iniciado el ciclo de tratamiento o han aparecido nuevas lesiones en ella, su médico debe replantearse la continuación de este tratamiento y considerar otras opciones terapéuticas.
Aplique una fina capa de Klisyri en la zona afectada de la cara o el cuero cabelludo una vez al día durante 5 días seguidos. Un sobre contiene pomada suficiente para cubrir la zona de tratamiento. No guarde el sobre abierto para usarlo otro día aunque todavía quede pomada.
Instrucciones de aplicación:
- Lávese las manos con agua y jabón antes de aplicarse la pomada.
- Lave la zona afectada con agua y jabón neutro y séquela suavemente.
- Abra un sobre nuevo cada vez que vaya a aplicarse este medicamento.
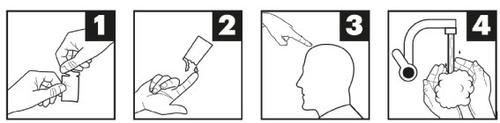
- Abra el sobre por la línea perforada (figura 1).
- Aplíquese un poco de pomada en la yema del dedo (figura 2).
- Extienda una capa fina de pomada de manera uniforme por toda la zona afectada (figura 3).
- Lávese las manos con agua y jabón inmediatamente después de aplicarse la pomada (figura 4).
- No se lave ni toque la zona tratada durante unas 8 horas. Una vez transcurrido este periodo, puede lavarse la zona tratada con agua y jabón neutro.
- No cubra la zona tratada con vendas después de haberse aplicado Klisyri.
- Repita los pasos anteriores cada día de tratamiento a la misma hora aproximadamente.
Si usa más Klisyri del que debe
Lávese la zona tratada con agua y jabón neutro. Consulte a su médico o farmacéutico si tiene reacciones cutáneas intensas.
Si olvidó usar Klisyri
Si olvida una dosis, aplíquese la pomada en cuanto se acuerde y después continúe con su pauta de tratamiento habitual. No se aplique la pomada más de una vez al día.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Después de usar este medicamento, puede experimentar efectos adversos en la zona de la piel donde se aplica la pomada. Estos efectos adversos pueden empeorar hasta 8 días después del inicio del tratamiento y, por lo general, desaparecen entre 2 y 3 semanas después de haberlo finalizado. Consulte a su médico si estos efectos adversos empeoran.
Efectos adversos más frecuentes en la zona tratada:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- enrojecimiento (eritema)
- descamación de la piel (exfoliación)
- costras (encostración)
- hinchazón
- pérdida de la capa superior de la piel (erosión, úlcera)
Otros posibles efectos adversos en la zona tratada:
Frecuentes(pueden afectar hasta a 1 de cada 10 personas)
- dolor (dolor a la palpación, escozor o sensación de ardor)
- picor (prurito)
- ampollas (vesículas, pústulas)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Klisyri
Mantener este medicamento fuera de la vista y del alcance de los niños.
No refrigerar o congelar.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y la etiqueta después de CAD. La fecha de caducidad es el último día del mes que se indica.
Para un solo uso. No reutilice los sobres una vez abiertos.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Klisyri
- El principio activo es tirbanibulina. Cada sobre contiene 2,5 mg de tirbanibulina en 250 mg de pomada. Cada gramo de pomada contiene 10 mg de tirbanibulina.
- Los demás componentes son propilenglicol y monoestearato de glicerol 40-55.
Aspecto del producto y contenido del envase
Cada sobre de Klisyri contiene 250 mg de pomada de color blanco o blanquecino. Cada caja contiene 5 sobres de polietileno/lámina de aluminio.
Titular de la autorización de comercialización
Almirall, S.A.
Ronda General Mitre, 151
08022 Barcelona
España
Responsable de la fabricación
Almirall Hermal GmbH Scholtzstrasse 3
21465 Reinbek
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Almirall, S.A. Tel: +34 93 291 30 00 | |
Fecha de la última revisión de este prospecto: 07/2021
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
- País de registro
- Precio medio en farmacia75.95 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a KLISYRI 10 MG/G POMADAForma farmacéutica: GEL, 7,5 mg/gPrincipio activo: MetronidazolFabricante: Laboratorios Vinas S.A.Requiere recetaForma farmacéutica: GEL, 7,5 mg/gPrincipio activo: MetronidazolFabricante: Laboratorios Galderma S.A.Requiere recetaForma farmacéutica: CREMA, 50 mg/gPrincipio activo: aciclovirFabricante: Aristo Pharma Iberia S.L.Requiere receta
Médicos online para KLISYRI 10 MG/G POMADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de KLISYRI 10 MG/G POMADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes








