INTRAROSA 6,5 MG OVULOS

Cómo usar INTRAROSA 6,5 MG OVULOS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Intrarosa 6,5 mg óvulos
prasterona
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas aunque tengan los mismos signos que usted, ya que puede perjudicarles.
- Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Intrarosa y para qué se utiliza
- Qué necesita saber antes de empezar a usar Intrarosa
- Cómo usar Intrarosa
- Posibles efectos adversos
- Conservación de Intrarosa
- Contenido del envase e información adicional
1. Qué es Intrarosa y para qué se utiliza
Intrarosa contiene el principio activo prasterona.
Para qué se utiliza Intrarosa
Intrarosa se utiliza para tratar a mujeres posmenopáusicas con atrofia vulvar y vaginal con síntomas de moderados a graves. Se utiliza para el alivio de los síntomas menopáusicos que afectan a la vagina, como sequedad o irritación. La causa es una disminución de los niveles de estrógenos en el organismo. Esto ocurre de forma natural después de la menopausia.
Cómo actúa Intrarosa
La prasterona corrige los síntomas y signos de la atrofia vulvar y vaginal al reemplazar los estrógenos que los ovarios de las mujeres producen normalmente antes de la menopausia. Se introduce dentro de la vagina, de manera que la hormona se libera en el lugar donde es necesaria. Esto puede aliviar las molestias vaginales.
2. Qué necesita saber antes de empezar a usar Intrarosa
El uso de terapia hormonal sustitutiva (THS) acarrea riesgos que deben tenerse en cuenta antes de decidir si se comienza a usar o se sigue utilizando dicha terapia.
La experiencia en el tratamiento de mujeres con menopausia prematura (debido a fallo ovárico o cirugía) es limitada. Si tiene menopausia prematura, los riesgos del uso de THS pueden ser diferentes. Consulte a su médico.
Antes de comenzar (o de reanudar) la THS, el médico le preguntará por sus antecedentes médicos y los de su familia. El médico puede decidir que es necesario hacerle una exploración física, que puede consistir en una exploración de las mamas y/o una exploración interna, si es necesario.
Una vez que haya empezado a tomar Intrarosa, debe acudir a su médico para revisiones periódicas (al menos cada 6 meses). En estas revisiones, puede comentar con su médico los beneficios y riesgos de continuar con Intrarosa.
Acuda periódicamente a realizarse exploraciones mamarias, según le recomiende su médico.
No use Intrarosa:
si cumple alguna de las condiciones siguientes. Si no está segura de alguno de los puntos siguientes, consulte a su médico antes de usar Intrarosa,
- Si tiene o ha tenido cáncer de mama, o sospecha que puede tenerlo;
- Si tiene o ha tenido cáncer sensible a los estrógenos, como cáncer del revestimiento del útero (endometrio), o sospecha que puede tenerlo;
- Si sufre cualquier sangrado vaginal de origen desconocido;
- Si tiene un engrosamiento excesivo del revestimiento del útero(hiperplasia endometrial) que no está siendo tratado;
- Si tiene o ha tenido un coágulo sanguíneo en una vena (trombosis), ya sea en las piernas (trombosis venosa profunda) o en los pulmones (embolia pulmonar);
- Si padece algún trastorno de la coagulación (como deficiencia de proteína C, proteína S o antitrombina);
- Si tiene o ha tenido recientemente una enfermedad provocada por coágulos sanguíneos en las arterias, como un infarto de miocardio, un accidente vascular cerebral o una angina de pecho.
- Si tiene o ha tenido una enfermedad del hígadoy los análisis de la función hepática todavía no se han normalizado;
- Si tiene un raro trastorno sanguíneo de transmisión hereditaria denominado «porfiria»;
- Si es alérgicaa la prasteronao a cualquiera de los demás ingredientes de este medicamento (indicados en la sección 6 «Contenido del envase e información adicional»).
Si alguno de estos trastornos aparece por primera vez mientras está usando Intrarosa, suspenda el tratamiento y consulte a su médico inmediatamente.
Advertencias y precauciones
Cuándo tener especial cuidado con Intrarosa
Informe a su médico si alguna vez ha tenido alguno de los siguientes problemas, antes de iniciar el tratamiento, ya que pueden reaparecer o empeorar durante el tratamiento con Intrarosa. Si es el caso, debe acudir a su médico con más frecuencia para revisiones:
- fibromas en el útero;
- crecimiento del revestimiento uterino fuera del útero (endometriosis) o antecedentes de engrosamiento excesivo del revestimiento uterino (hiperplasia endometrial);
- antecedentes de formación de coágulos sanguíneos (ver «Coágulos sanguíneos en una vena (trombosis)»);
- aumento del riesgo de sufrir un cáncer sensible a los estrógenos (como haber tenido una madre, hermana o abuela que hayan padecido un cáncer de mama);
- tensión arterial alta;
- trastornos del hígado, como un tumor hepático benigno;
- diabetes;
- cálculos en la vesícula biliar;
- migraña o dolores de cabeza (fuertes);
- una enfermedad del sistema inmunitario que afecta a muchos órganos del cuerpo (lupus eritematoso sistémico, LES);
- epilepsia;
- asma;
- una enfermedad que afecta a la membrana del tímpano y a la audición (otosclerosis);
- un nivel muy alto de grasa en la sangre (triglicéridos);
- retención de líquidos debido a problemas de corazón o de riñón.
Deje de tomar Intrarosa y acuda al médico inmediatamente
Si observa cualquiera de los siguientes síntomas al utilizar la THS:
- cualquiera de los trastornos mencionados en la sección «No utilice Intrarosa»;
- coloración amarillenta de la piel o el blanco de los ojos (ictericia). Pueden ser síntomas de una enfermedad del hígado;
- si se queda embarazada;
- un gran aumento de la presión arterial (con síntomas como dolor de cabeza, cansancio, mareos);
- dolor de cabeza tipo migraña, que puede presentarse por primera vez;
- si nota síntomas de un coágulo sanguíneo, como:
- inflamación con dolor y enrojecimiento de las piernas;
- dolor súbito en el pecho;
- dificultad para respirar.
Para más información, ver «Coágulos sanguíneos en una vena (trombosis)».
Nota:Intrarosa no es un anticonceptivo. Si han transcurrido menos de 12 meses desde la última menstruación o si tiene menos de 50 años, es posible que tenga que seguir utilizando métodos anticonceptivos para no quedarse embarazada. Pida consejo a su médico.
THS y cáncer
Intrarosa no se ha estudiado en mujeres con diagnóstico actual o antecedentes de cáncer.
Engrosamiento excesivo del revestimiento del útero (hiperplasia endometrial) y cáncer del revestimiento del útero (cáncer de endometrio)
La administración prolongada de THS solo con estrógenos en comprimidos puede aumentar el riesgo de desarrollar cáncer del revestimiento del útero (endometrio). Intrarosa no estimula el crecimiento del endometrio, como demuestra la atrofia del revestimiento del útero en todas las mujeres tratadas con Intrarosa durante un año en los ensayos clínicos.
No está claro si existe algún riesgo cuando Intrarosa se utiliza para tratamientos a largo plazo (más de un año). Sin embargo, se ha demostrado que la absorción de Intrarosa en la sangre es muy pequeña, por lo que no es necesario añadir un progestágeno.
Si presenta sangrado o manchado vaginal, normalmente no es preocupante, pero debe concertar una visita con su médico. Puede ser una señal de que el endometrio se ha engrosado.
Los riesgos siguientes se relacionan con los medicamentos de THS que circulan en la sangre. Ahora bien, Intrarosa se utiliza para el tratamiento local de la vagina y la absorción en la sangre es muy pequeña. Es menos probable que los trastornos mencionados a continuación empeoren o reaparezcan durante el tratamiento con Intrarosa, pero debe acudir a su médico si está preocupada.
Cáncer de mama
Los datos disponibles indican que la THS que combina estrógenos-progestágenos, y posiblemente también la THS con solo estrógenos, aumenta el riesgo de cáncer de mama. El riesgo adicional depende del tiempo que dure la THS. El aumento del riesgo se hace patente después de unos años de THS. Sin embargo, retorna a la normalidad a los pocos años (como máximo 5) de haber suspendido el tratamiento.
- Examine sus mamas regularmente. Acuda al médico si nota cualquier cambio, como:
- hoyuelos en la piel;
- cambios en los pezones;
- cualquier bulto que pueda ver o notar.
Además, le recomendamos que siga programas de detección precoz con mamografías cuando se le ofrezcan.
Cáncer de ovario
El cáncer de ovario es raro, mucho más raro que el cáncer de mama. El uso de THS con solo estrógenos se ha asociado con un ligero aumento del riesgo de cáncer de ovario.
El riesgo de cáncer de ovario varía con la edad. Por ejemplo, en mujeres de 50 a 54 años que no reciben THS, se diagnosticarán alrededor de 2 casos de cáncer de ovario por cada 2000 mujeres en un periodo de 5 años. En mujeres que han recibido THS durante 5 años, se producirán alrededor de 3 casos por cada 2000 usuarias de THS (es decir, alrededor de 1 caso adicional).
Se han comunicado casos raros de cáncer de ovario y de mama en mujeres tratadas con 6,5 mg de prasterona durante 52 semanas.
Efecto de la THS sobre el corazón y la circulación
Intrarosa no se ha estudiado en mujeres con antecedentes de enfermedades tromboembólicas, hipertensión no controlada o enfermedades del corazón.
Coágulos sanguíneos en una vena (trombosis)
El riesgo de coágulos sanguíneos en las venas es aproximadamente de 1,3 a 3 veces superior en las usuarias de THS frente a las no usuarias, especialmente en el primer año de tratamiento.
Los coágulos sanguíneos pueden ser graves y si uno de ellos llega a los pulmones, puede provocar dolor en el pecho, dificultad para respirar, síncope o incluso la muerte.
La probabilidad de padecer coágulos sanguíneos será mayor con el aumento de la edad y si interviene uno de los siguientes factores. En caso de que alguna de estas situaciones pueda aplicarse a usted, informe a su médico:
- no puede caminar durante mucho tiempo debido a una intervención de cirugía mayor, lesión o enfermedad (ver también la sección 3, Si necesita someterse a una intervención quirúrgica);
- tiene un sobrepeso importante (IMC > 30 kg/m2);
- sufre un problema de coagulación sanguínea que necesita un tratamiento prolongado con medicación para prevenir los coágulos;
- algún familiar cercano ha tenido alguna vez un coágulo sanguíneo en piernas, pulmones u otros órganos;
- padece lupus eritematoso sistémico (LES);
- tiene cáncer.
Para ver los síntomas que provoca un coágulo sanguíneo, consulte la sección «Deje de tomar Intrarosa y acuda a su médico inmediatamente».
En los ensayos clínicos no se ha observado trombosis venosa profunda con prasterona intravaginal y el único caso de embolia pulmonar observado indica una incidencia menor con Intrarosa que en el grupo de placebo.
Comparación
Se calcula que, durante un periodo de 5 años, un promedio de entre 4 y 7 de cada 1000 mujeres de alrededor a los 50 años de edad que no reciben THS presentarán un coágulo sanguíneo en una vena.
Enfermedad cardíaca (infarto de miocardio)/Hipertensión
En las mujeres que reciben terapia solo con estrógenos no aumenta el riesgo de sufrir una enfermedad del corazón.
Accidente cerebrovascular
El riesgo de sufrir un accidente vascular cerebral es aproximadamente 1,5 veces mayor en las usuarias de THS que en las no usuarias. El número de casos adicionales de accidente cerebrovascular debido al uso de THS aumenta con la edad.
No se han observado casos de accidente cerebrovascular con Intrarosa en los ensayos clínicos.
Comparación
Se calcula que, durante un periodo de 5 años, un promedio de 8 de cada 1000 mujeres, de alrededor de 50 años, que no reciben THS sufrirán un accidente vascular cerebral. En las mujeres de alrededor de 50 años que están recibiendo THS, el número de casos será de 11 por cada 1000 usuarias durante un periodo de 5 años (es decir, 3 casos adicionales).
Otras condiciones
- La THS no previene la pérdida de memoria. Existen algunos indicios de un mayor riesgo de pérdida de memoria en mujeres que comenzaron a usar THS después de los 65 años. Pida consejo a su médico.
- Puede presentar flujo vaginal debido a que la «base de grasa » se funde, y esto se añade al aumento de las secreciones vaginales debido al tratamiento. Si se produce flujo vaginal, no es necesario interrumpir la administración de Intrarosa.
- Intrarosa puede alterar los preservativos, diafragmas y capuchones cervicales de látex.
- Si tiene una infección vaginal, necesitará recibir un ciclo de antibióticos antes de tomar Intrarosa.
Niños y adolescentes
Intrarosa solo se utiliza en mujeres adultas.
Otros medicamentos e Intrarosa
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
No se dispone de datos de eficacia y seguridad en mujeres tratadas actualmente con tratamiento hormonal como: andrógenos, THS (estrógenos solos o combinados con progestágenos).
No se recomienda el uso de Intrarosa en combinación con THS (tratamiento con solo estrógenos, estrógenos-progestágenos o tratamiento con andrógenos) o con estrógenos vaginales.
Embarazo, lactancia y fertilidad
Embarazo y lactancia
Intrarosa solo debe utilizarse en mujeres posmenopáusicas. Si se queda embarazada, deje de tomar Intrarosa y consulte a su médico.
Fertilidad
Intrarosa está contraindicada en mujeres en edad fértil. No se sabe si este medicamento afecta a la fertilidad.
Conducción y uso de máquinas
Intrarosa no afecta generalmente a la capacidad para conducir o utilizar máquinas.
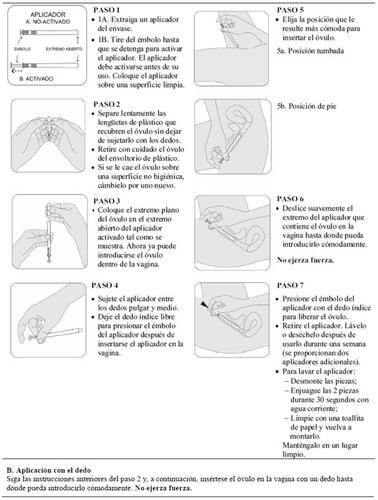
3. Cómo usar Intrarosa
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Su médico intentará recetarle la dosis más baja para tratar sus síntomas durante el período más corto como sea necesario. Hable con su médico si cree que esta dosis es excesiva o insuficiente.
Qué cantidad debe usar
Utilice un óvulo una vez al día, al acostarse.
Cómo usar Intrarosa
Introduzca el óvulo en la vagina con el dedo o con un aplicador suministrado en el envase.
Lea cuidadosamente las instrucciones de uso de Intrarosa al final del prospecto antes de utilizar este medicamento.
Durante cuánto tiempo se debe usar
Una vez iniciado su uso, acuda al médico al menos cada 6 meses para comprobar si tiene que seguir utilizando Intrarosa.
Si usa más Intrarosa de lo que debe
Se recomienda una ducha vaginal.
Si olvidó usar Intrarosa
Si olvidó usar un óvulo, insértese uno en cuanto se acuerde. Ahora bien, si faltan menos de 8 horas para la dosis siguiente, sáltese el óvulo olvidado.
No use dos óvulos para compensar la dosis olvidada.
Si necesita someterse a una intervención quirúrgica
Si va a someterse a una intervención quirúrgica, informe al cirujano de que está usando Intrarosa. Es posible que tenga que dejar de usar Intrarosa entre 4 y 6 semanas antes de la operación para reducir el riesgo de un coágulo de sangre (ver sección 2, «Coágulos de sangre en una vena (trombosis)»). Pregunte a su médico cuándo puede empezar a usar Intrarosa de nuevo.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Las siguientes enfermedades se observan con más frecuencia en mujeres que utilizan medicamentos de THS que circulan en la sangre, en comparación con las mujeres que no utilizan THS. Estos riesgos se aplican menos a los tratamientos con estrógenos administrados por vía vaginal:
- cáncer de mama;
- cáncer de ovarios;
- coágulos sanguíneos en las venas de las piernas o los pulmones (tromboembolismo venoso);
- accidente cerebrovascular;
- probable pérdida de memoria si se inicia la THS con más de 65 años de edad.
Para más información sobre estos efectos adversos, consulte la sección 2.
El efecto adverso comunicado con más frecuencia en los estudios clínicos fue el flujo vaginal. Esto se debe probablemente a que la grasa dura se funde y esto se añade al aumento esperado de las secreciones vaginales causado por el tratamiento. El flujo vaginal no obliga a interrumpir la administración de Intrarosa.
También se comunicaron los siguientes efectos adversos:
- frecuentes (puede afectar hasta 1 de cada 10 personas): citología vaginal anormal (en la mayoría de los casos, ASCUS o LGSIL), fluctuaciones del peso (aumento o disminución);
- poco frecuentes (puede afectar hasta 1 de cada 100 personas): pólipos cervicales o uterinos benignos, masa mamaria benigna.
Se han notificado los siguientes efectos adversos con THS que contienen estrógenos, pero no con Intrarosa, durante los ensayos clínicos:
- enfermedad de la vesícula biliar
- diversos trastornos cutáneos:
- pigmentación de la piel, especialmente en la cara y el cuello, lo que se conoce como «paño del embarazo» (cloasma)
- nódulos cutáneos rojos y dolorosos (eritema nodular)
- erupción con úlceras o enrojecimientos en forma de diana (eritema multiforme)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Intrarosa
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y el blíster después de EXP. La fecha de caducidad es el último día del mes que se indica.
Conservar por debajo de 30 °C.
No congelar.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Intrarosa
- El principio activo es la prasterona. Cada óvulo contiene 6,5 mg de prasterona.
- El otro componente es la grasa dura (adeps solidus).
Aspecto del producto y contenido del envase
Intrarosa es un óvulo de color blanco o blanquecino, con forma de bala, de aproximadamente 28 mm de longitud y 9 mm de diámetro en su extremo más ancho.
El aplicador es de LDPE y colorante al 1 % (dióxido de titanio).
Se presenta en envases blíster que contienen 28 óvulos con 6 aplicadores.
Titular de la autorización de comercialización
Endoceutics S.A.
Rue Belliard 40
1040 Bruselas
Bélgica
Responsable de la fabricación
Basic Pharma Manufacturing B.V.
Burgemeester Lemmensstraat 352
6163 JT Geleen
Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización: België/Belgique/Belgien, Luxembourg/Luxemburg, Nederland Theramex Belgium BVBA Tél/Tel: + 32 (0) 28088080 |
Deutschland, Österreich Theramex Germany GmbH Tel: + 49 (0) 32 2122 490 37 |
España Theramex Ireland Limited Tel: + 34 911 143 487 |
France Theramex France S.A.S. Tél: + 33 (0) 800100350 |
Italia Theramex Italy S.r.l. Tel: + 39 02 81480024 |
Polska Theramex Poland sp. z o.o. Tel.: + 48 (0) 22 30 77 166 |
Portugal Tecnimede - Sociedade Técnico-Medicinal, S.A. Tel: + 351 210 414 100 |
United Kingdom (Northern Ireland), Ireland, Malta Theramex UK Limited Tel: + 44 (0) 3330096795 |
Danmark, Ísland, Norge, Suomi/Finland, Sverige Avia Pharma AB Sverige/Svíþjóð/Ruosti Tlf/Sími/Tlf/Puh/Tel: + 46 (0) 8 544 900 22 Ceská republika, Eesti,Ελλ?δα, Hrvatska,Κ?προ, Latvija,Lietuva, Magyarország, România, Slovenija, Slovenská republika Theramex Ireland Limited Tel/Te?./Τηλ: + 353 (0) 15138855 Fecha de la última revisión de este prospecto 12/2023. Otras fuentes de información La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu. Instrucciones de uso de Intrarosa Cómo debo utilizar Intrarosa
Antes de empezar
|
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a INTRAROSA 6,5 MG OVULOSForma farmacéutica: COMPRIMIDO, 20 mgPrincipio activo: bazedoxifeneFabricante: Pfizer Europe Ma EeigRequiere recetaForma farmacéutica: COMPRIMIDO, 20 mgPrincipio activo: bazedoxifeneFabricante: Pfizer Europe Ma EeigRequiere recetaForma farmacéutica: COMPRIMIDO, 20 mgPrincipio activo: bazedoxifeneFabricante: Pfizer Europe Ma EeigRequiere receta
Médicos online para INTRAROSA 6,5 MG OVULOS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de INTRAROSA 6,5 MG OVULOS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes