INCRELEX 10 mg/ml SOLUCION INYECTABLE

Cómo usar INCRELEX 10 mg/ml SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
INCRELEX 10 mg/ml solución inyectable
Mecasermina
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas aunque tengan los mismos síntomas, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es INCRELEX y para qué se utiliza
- Qué necesita saber antes de empezar a usar INCRELEX
- Cómo usar INCRELEX
- Posibles efectos adversos
- Conservación de INCRELEX
- Contenido del envase e información adicional
1. Qué es INCRELEX y para qué se utiliza
- INCRELEX es una solución líquida que contiene mecasermina, que es un factor de crecimiento insulínico tipo 1 (IGF-1) sintético, que es similar al IGF-1 que fabrica su organismo.
- Se utiliza para tratar a niños y adolescentes de 2 a 18 años que son demasiado bajos para su edad porque su organismo no puede fabricar suficiente IGF-1. Esta enfermedad se denomina déficit primario de IGF-1.
2. Qué necesita saber antes de empezar a usar INCRELEX
No use INCRELEX
- Si usted actualmente tiene cualquier tumor o crecimiento anormal, ya sea canceroso o no canceroso
- Si ha tenido cáncer en el pasado
- Si presenta cualquier patología que pueda incrementar el riesgo de desarrollar cáncer
- Si usted es alérgico (hipersensible) a la mecasermina o a cualquiera de los otros ingredientes de este medicamento (incluidos en la sección 6).
- En neonatos o recién nacidos prematuros porque contiene alcohol bencílico.
Advertencias y precauciones
Existe un incremento del riesgo de desarrollar tumores (tanto cancerosos como no cancerosos) en niños y adolescentes tratados con INCRELEX. Dado que mecasermina puede intervenir en el desarrollo del cáncer, informe a su médico inmediatamente, si durante el tratamiento, o después del mismo, aparece cualquier nuevo tumor, lesión cutánea o síntoma inesperado.
Consulte a su médico o farmacéutico antes de empezar a usar INCRELEX
- Si usted sufre una desviación lateral de la columna (escoliosis). Conviene supervisar la progresión de la escoliosis.
- Si desarrolla cojera o dolor en la rodilla o cadera
- Si usted presenta un aumento del tamaño de las amígdalas (hipertrofia amigdalar). Conviene someterse a reconocimientos periódicos.
- Si usted presenta síntomas que indiquen un aumento de la presión en el cerebro (hipertensión intracraneal), como cambios visuales, dolor de cabeza, náuseas y/o vómitos; consulte a su médico.
- Si usted presenta una reacción localizada en el lugar de inyección o una reacción alérgica generalizada a INCRELEX. Acuda al médico lo antes posible si usted desarrolla una erupción cutánea localizada. Busque asistencia médica inmediatamente si usted tiene una reacción alérgica generalizada (urticaria, problemas para respirar, desvanecimiento o colapso y sensación general de malestar).
- Si usted ha dejado de crecer (se ha producido el cierre de las placas de crecimiento óseo). En este caso INCRELEX no puede ayudarle a crecer y no debe utilizarlo.
Niños de menos de 2 años
No se ha estudiado el uso de este medicamento en niños menores de 2 años, por lo tanto, no está recomendado.
Otros medicamentos e INCRELEX
Informe a su médico o farmacéutico si está tomando o ha tomado recientemente, o podría tener que tomar cualquier otro medicamento.
Informe a su médico especialmente si usted se trata con insulina u otros medicamentos antidiabéticos.
Puede ser necesario ajustar la dosis de estos medicamentos.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Para todas las mujeres en edad fértil se recomienda una prueba de embarazo negativa antes de iniciar el tratamiento con mecasermina. También se recomienda que todas las mujeres en edad fértil utilicen medidas anticonceptivas adecuadas durante el tratamiento.
El tratamiento con mecasermina debe suspenderse si se produce un embarazo.
Mecasermina no debe administrarse a una madre en lactancia.
Conducción y uso de máquinas
Mecasermina puede causar hipoglucemia (efecto adverso muy frecuente, ver sección 4) que puede afectar a su capacidad para conducir y utilizar máquinas, ya que su capacidad para concentrarse o reaccionar puede estar reducida.
Usted debe evitar cualquier actividad de alto riesgo (p. ej. conducir, etc) en las 2-3 horas siguientes a la administración de la dosis, especialmente al principio del tratamiento con INCRELEX y hasta que se haya encontrado una dosis de INCRELEX que no produzca efectos adversos que hagan de estas actividades un riesgo.
INCRELEX contiene alcohol bencílico y sodio
INCRELEX contiene alcohol bencílico como conservante que puede provocar reacciones tóxicas y alérgicas en lactantes y en niños de hasta 3 años de edad.
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por vial, lo que se considera esencialmente “exento de sodio”.
3. Cómo usar INCRELEX
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico.
Consulte a su médico o farmacéutico si tiene dudas.
La dosis normal es de 0,04 a 0,12 mg por kg de peso del paciente administrada dos veces al día.
Consulte las “Instrucciones de uso” que figuran al final de este prospecto.
Inyecte INCRELEX justo debajo de su piel un poco antes o después de una comida o tentempié, ya que puede tener efectos hipoglucemiantes tipo insulina y por tanto, puede disminuir los niveles de azúcar en sangre (ver hipoglucemia en la sección 4). No se inyecte la dosis de INCRELEX si usted no puede comer por cualquier motivo. No intente compensar la dosis omitida administrando el doble de dosis la siguiente vez. La siguiente dosis debe administrarse como siempre, con una comida o tentempié.
Inyecte INCRELEX justo debajo de su piel en algún punto del brazo, el muslo, la zona del estómago (abdomen) o las nalgas. No inyecte nunca en una vena o en un músculo. Cambie el lugar de inyección en cada ocasión.
No use INCRELEX si no es transparente e incoloro.
El tratamiento con mecasermina es un tratamiento a largo plazo. Consulte a su médico para más información.
Si usa más INCRELEX del que debiera
Mecasermina, como la insulina, puede disminuir los niveles de azúcar en sangre (ver hipoglucemia en la sección 4).
Si se ha inyectado más INCRELEX del recomendado, contacte con su médico inmediatamente.
Una sobredosis aguda puede provocar hipoglucemia (baja concentración de glucosa en sangre).
El tratamiento de la sobredosis aguda de mecasermina debe concentrarse en invertir la hipoglucemia. Debe ingerirse comida o bebidas azucaradas. Si el paciente no está lo suficientemente consciente para tomar bebidas azucaradas, puede ser necesaria una inyección intramuscular de glucagón para invertir la hipoglucemia. Su médico o enfermera le enseñarán cómo inyectar el glucagón.
La sobredosis prolongada puede provocar un aumento de tamaño de ciertas partes del cuerpo (p. ej., las manos, pies, partes de la cara) o un crecimiento excesivo de todo el cuerpo. Si sospecha de una sobredosis prolongada, contacte con su médico inmediatamente.
Si olvidó usar INCRELEX
No tome una dosis doble para compensar las dosis olvidadas.
Si se ha saltado una dosis, la siguiente dosis no debe ser mayor para compensar. La siguiente dosis debe administrarse como siempre, con una comida o tentempié.
Si interrumpe el tratamiento con INCRELEX
Una interrupción o finalización prematura del tratamiento con mecasermina puede reducir el éxito de la terapia de crecimiento. Pregunte a su médico antes de interrumpir el tratamiento.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto.
Los efectos adversos más frecuentemente notificados con mecasermina son: bajo azúcar en sangre (hipoglucemia), vómitos, reacciones en el lugar de la inyección, dolor de cabeza e infecciones del oído medio. También se han notificado reacciones alérgicas graves con INCRELEX. Si usted experimenta cualquiera de estos efectos, siga el consejo que se da para cada efecto en las siguientes secciones.
Frecuencia no conocida (la frecuencia no puede estimarse a partir de los datos disponibles)Tumores cancerosos y no cancerosos
En pacientes tratados con INCRELEX se ha notificado un incremento en la aparición de tumores cancerosos y no cancerosos. El riesgo de desarrollar dichos tumores puede ser mayor si INCRELEX se utiliza para una patología distinta a la que se indica en la sección 1 o a una dosis mayor a la recomendada en la sección 3.
Reacciones alérgicas graves (anafilaxis)
Tras el uso de mecasermina se ha comunicado urticaria generalizada, dificultad al respirar, somnolencia, inflamación de la cara y/o garganta. Si desarrolla una reacción alérgica grave, debe interrumpir INCRELEX inmediatamente y buscar asistencia médica urgente.
También se han notificado reacciones alérgicas locales en el lugar de la inyección (picor, urticaria).
Caída de cabello (alopecia)
Con el uso de mecasermina también se ha notificado la caída de cabello.
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
Bajo azúcar en sangre (hipoglucemia)
Mecasermina puede disminuir los niveles de azúcar en sangre. Los signos de bajo azúcar son: somnolencia, cansancio, inquietud, hambre, irritabilidad, problemas de concentración, sudores, náuseas y latidos rápidos o irregulares.
La hipoglucemia grave puede causar inconsciencia, convulsiones/accesos epilépticos o muerte. Interrumpa INCRELEX inmediatamente y busque asistencia médica urgente si usted desarrolla convulsiones/accesos epilépticos o inconsciencia.
Si usted toma INCRELEX, debe evitar participar en actividades de riesgo elevado (como actividad física intensa) en las 2 a 3 horas siguientes a la inyección de INCRELEX, especialmente al inicio del tratamiento con INCRELEX.
Antes de comenzar el tratamiento con INCRELEX, el médico o la enfermera le explicarán cómo tratar la hipoglucemia. Usted debe tener siempre a mano una fuente de azúcar, como zumo de naranja, gel de glucosa, dulces o leche por si aparecen síntomas de hipoglucemia. En caso de hipoglucemia grave, si usted no responde y no puede tomar una bebida azucarada, debe administrarse una inyección de glucagón. El médico o la enfermera le enseñarán cómo administrar la inyección. La inyección de glucagón aumenta la concentración de glucosa en sangre. Es importante que usted siga una dieta equilibrada que incluya, además de alimentos azucarados, proteínas y grasas como carne y queso.
Hipertrofia en el lugar de inyección (el tejido en el lugar de la inyección aumenta de tamaño) y hematomas
Esto se puede evitar cambiando el lugar de inyección en cada ocasión (rotación del lugar de inyección).
Aparato digestivo
Con el tratamiento con mecasermina se han producido casos de vómitos y dolor en la parte superior de la barriga.
Infecciones
En niños en tratamiento con mecasermina se han observado infecciones del oído medio.
Sistema músculo-esquelético
Con el tratamiento con mecasermina ha habido casos de dolor en articulaciones y dolor en extremidades.
Sistema nervioso
Con el tratamiento con mecasermina se han producido casos de dolor de cabeza.
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
Convulsiones
Con el tratamiento con mecasermina se han observado casos de convulsiones (accesos).
Con el tratamiento con mecasermina también se han comunicado casos de mareo y temblores.
Anomalías cardíacas
Con el tratamiento con mecasermina se han comunicado casos de pulso acelerado y ruidos cardíacos anómalos.
Aumento de azúcar en sangre (hiperglucemia)
Con el tratamiento con mecasermina también se ha observado aumento de azúcar en sangre.
Aumento de las amígdalas/adenoides
Mecasermina puede aumentar el tamaño de las amígdalas/adenoides. Entre los signos de un aumento del tamaño de las amígdalas/adenoides se incluyen: ronquidos, dificultad para respirar o tragar, apnea del sueño (un trastorno en el que se producen episodios breves de suspensión de la respiración durante el sueño) o presencia de líquido en el oído medio, además de infecciones del oído. La apnea del sueño puede provocar somnolencia diurna excesiva. Acuda al médico si estos síntomas resultan molestos. El médico debe examinar las amígdalas con regularidad.
Aumento del tamaño del timo
En el tratamiento con mecasermina se ha observado aumento del tamaño del timo (un órgano especializado del sistema inmune).
Papiledema
Durante el tratamiento con mecasermina el médico o un óptico puede observar una inflamación de la parte posterior del ojo (debida al aumento de presión en el cerebro).
Hipoacusia (pérdida de audición)
Con el tratamiento con mecasermina se ha observado hipoacusia (pérdida de audición), dolor en el oído y líquido en el oído medio. Informe a su médico si usted desarrolla problemas de audición.
Empeoramiento de la escoliosis (producido por un crecimiento rápido)
Si usted padece escoliosis, será necesario que se someta a exámenes frecuentes para comprobar si ha aumentado la desviación de la columna. En el tratamiento con mecasermina también se han observado casos de dolor muscular.
Aparato reproductor
Con el tratamiento con mecasermina se han observado casos de aumento mamario.
Aparato digestivo
En el tratamiento con mecasermina se han dado casos de dolor de barriga.
Cambios cutáneos y capilares
Con el tratamiento con mecasermina se han observado casos de engrosamiento de la piel, lunares, y alteración de la textura capilar.
Reacciones en el lugar de la inyección
Las reacciones comunicadas con el tratamiento con mecasermina incluyen dolor, irritación, hemorragia, hematoma, enrojecimiento y endurecimiento. Las reacciones del lugar de inyección se pueden evitar cambiando el lugar de inyección en cada ocasión (rotación del lugar de inyección).
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas)
Aumento de la presión cerebral (Hipertensión intracraneal)
INCRELEX puede provocar a veces un aumento temporal de la presión cerebral. Entre los posibles síntomas de la hipertensión intracraneal se incluyen los cambios visuales, el dolor de cabeza, las náuseas y/o vómitos. Informe a su médico inmediatamente si usted padece cualquiera de estos síntomas. Su médico comprobará si se debe a una hipertensión intracraneal. Si este fuera el caso, puede que su médico decida suspender o reducir temporalmente el tratamiento con mecasermina. Mecasermina se puede volver a administrar cuando haya pasado el episodio.
Anomalías cardíacas
En algunos pacientes tratados con mecasermina, el examen del corazón (ecocardiograma) ha puesto de manifiesto un aumento de tamaño del músculo cardíaco y anomalías en el funcionamiento de las válvulas cardíacas. Su médico puede hacerle un ecocardiograma antes, durante y después del tratamiento con mecasermina.
Reacciones en el lugar de la inyección
Con el tratamiento con INCRELEX se han comunicado reacciones que incluyen erupción cutánea, hinchazón y bultos de grasa. Las reacciones del lugar de inyección se pueden evitar cambiando el lugar de inyección en cada ocasión (rotación del lugar de inyección).
Aumento de peso
Con el tratamiento con mecasermina se han comunicado casos de aumento de peso.
Otros efectos adversos poco frecuentes con el tratamiento con mecasermina incluyen depresión, nerviosismo.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero,
incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede
comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V.Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de INCRELEX
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y la etiqueta después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 ºC y 8 °C). No congelar.
Mantener el vial perfectamente cerrado para protegerlo de la luz.
Después de usarse por primera vez, el vial puede ser almacenado hasta 30 días entre 2 ºC y 8 ºC.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de INCRELEX
- El principio activo es la mecasermina. Un mililitro contiene 10 mg de mecasermina. Cada vial contiene 40 mg de mecasermina.
- Los demás componentes son: alcohol bencílico, cloruro sódico, polisorbato 20, ácido acético glacial, acetato sódico y agua para preparaciones inyectables (ver sección 2 “INCRELEX contiene alcohol bencílico y sodio”).
Aspecto del producto y contenido del envase
INCRELEX es una solución inyectable (inyección) transparente e incolora suministrada en un vial de vidrio cerrado con un tapón y una cápsula. El vial contiene 4 ml de solución.
Tamaño de envase de 1 vial.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
Ipsen Pharma
65, quai Georges Gorse
92100 Boulogne-Billancourt
Francia
Responsable de la fabricación:
Beaufour Ipsen Industrie
Rue d'Ethe Virton
28100 Dreux
Francia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien, Luxembourg/Luxemburg Ipsen NV Guldensporenpark 87 B-9820 Merelbeke België /Belgique/Belgien Tél/Tel: + 32 9 243 96 00 | Italia Ipsen SpA Via del Bosco Rinnovato n.6 Milanofiori Nord Palazzo U7 20090 Assago (Mi) Tel: + 39 - 02 - 39 22 41 |
România, ???????? Ipsen Pharma Str. Grigore Alexandrescu nr 59, cladirea HQ Sector 1, 010623, Bucuresti, România Tel/ ???.: + 40 (021) 231 27 20 | Ceská Republika Ipsen Pharma, s.r.o. Olbrachtova 2006/9 140 00 Praha 4 Tel: + 420 242 481 821 |
Danmark,Norge, Suomi/Finland, Sverige, Ísland Institut Produits Synthèse (IPSEN) AB Kista Science Tower Färögatan 33 SE - 164 51 Kista Sverige/Ruotsi/Svíþjóð Tlf/Puh/Tel/Sími: +46 8 451 60 00 | Deutschland, Österreich Ipsen Pharma GmbH Einsteinstrasse 174 D-81677 München Tel.: +49 89 262043289 |
Eesti Centralpharma Communications OÜ Selise 26-11, 13522, Tallinn Tel: +372 6015540 | Ελλ?δα, Κ?προς, Malta Ipsen Μονοπρ?σωπη EΠΕ Αγ. Δημητρ?ου 63 ?λιμος GR-17456 Αθ?να Ελλ?δα Τηλ: + 30 - 210 - 984 3324 |
España Ipsen Pharma, S.A. Torre Realia, Plaza de Europa, 41 - 43 08908 L’Hospitalet de Llobregat Barcelona Tel: + 34 - 936 - 858 100 | France Ipsen Pharma 65 quai Georges Gorse F-92100 Boulogne-Billancourt Tél: + 33 - 1 - 58 33 50 00 |
Latvija Ipsen Pharma parstavnieciba Latvija Kalnciema iela 33-5 Riga LV 1046 Tel: +371 67622233 | Lietuva, Hrvatska Ipsen Pharma Lietuvos filialas ojo Forto 47 LT-48100 Kaunas Tel. + 370 37 337854 |
Magyarország Ipsen Pharma SAS Magyarországi Kereskedelmi Képviselet Árbóc utca 6. H-1133 Budapest Tel: +36 1 555 5930 | Nederland Ipsen Farmaceutica B.V. Taurusavenue 33 B NL-2132 LS Hoofddorp Tel: + 31 23 55 41 600 |
Polska Ipsen Poland Sp. z o.o. Al. Jana Pawla II 29 PL-00-867 Warszawa Tel.: + 48 (0) 22 653 68 00 | Portugal Ipsen Portugal - Produtos Farmacêuticos S.A. Alameda Fernão Lopes, no 16A-1oB P-1495 - 190 Algés Tel: + 351 - 21 - 412 3550 |
Slovenija PharmaSwiss d.o.o Brodišce 32 SI-1236 Trzin Tel: + 386 1 236 47 00 | United Kingdom Ipsen Ltd. 190 Bath Road Slough, Berkshire SL1 3XE Tel: + 44 - (0)1753 - 62 77 00 |
Ireland Ipsen Pharmaceuticals Ltd. Blanchardstown Industrial Park Blanchardstown IRL-Dublin 4 Tel: + 353 - 1 - 8098200 | Slovenská republika Liek s.r.o. Hviezdoslavova 19 SK-903 01 Senec Tel: + 421 245 646 322 |
Fecha de la última revisión de este prospecto:
Este medicamento se ha autorizado en "circunstancias excepcionales".
Esta modalidad de aprobación significa que debido a la rareza de su enfermedad no ha sido posible obtener información completa de este medicamento.
La Agencia Europea de Medicamentos revisará anualmente la información nueva del medicamento que pueda estar disponible, y este prospecto se actualizará cuando sea necesario.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu. También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
En la página web de la Agencia Europea de Medicamentos puede encontrarse este prospecto en todas
las lenguas de la Unión Europea/Espacio Económico Europeo
<---------------------------------------------------------------------------------------------------------------------->
INSTRUCCIONES DE USO
INCRELEX debe administrarse usando agujas y jeringas estériles desechables que puede proporcionarle su médico/farmacéutico o/enfermera. Hay que utilizar jeringas de pequeño volumen que permitan extraer del vial la dosis prescrita con una exactitud razonable.
Preparación de la dosis
- Lávese las manos antes de preparar INCRELEX para la inyección.
- Use una aguja y una jeringa desechables nuevas cada vez que administre una dosis. No use las jeringas y las agujas más de una vez. Deshágase de ellas de manera adecuada en un contenedor para objetos punzantes (por ejemplo, un contenedor para residuos biológicos peligrosos), un recipiente de plástico rígido (por ejemplo, una botella de detergente) o un recipiente de metal (como una lata de café vacía) No comparta nuncalas agujas y las jeringas.
- Compruebe que el líquido es transparente e incoloro. No lo utilice después de la fecha de caducidad (que aparece en la etiqueta después de CAD y se refiere al último día del mes), o si está turbio u observa que contiene partículas. Si un vial se congela, deshágase de él adecuadamente. Pregunte a su farmacéutico cómo deshacerse de los medicamentos que ya no utiliza.
- Si va a usar un vial nuevo, quite la tapa de protección. No quite el tapón de caucho.
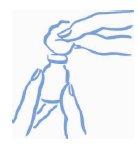
- Frote el tapón de caucho del vial con un algodón empapado en alcohol para evitar que el vial se contamine con los gérmenes que pudieran introducirse al insertar repetidas veces la aguja (ver la figura 1).
|
Figura 1: Frote la parte superior del tapón con alcohol |
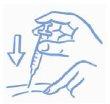
- Antes de introducir la aguja en el vial, tire del émbolo hacia atrás para introducir en la jeringa una cantidad de aire equivalente a la dosis prescrita. Clave la aguja a través del tapón de caucho del vial y empuje el émbolo para inyectar aire dentro del vial (ver la figura 2).
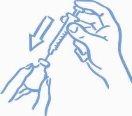
Figura 2: Inyecte
aire en el vial
- Deje la jeringa dentro del vial y déles la vuelta a ambos. Sujete la jeringa y el vial con fuerza (ver la figura 3).
Figura 3: Prepárese para la extracción |
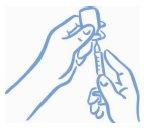
- Asegúrese de que la punta de la aguja está dentro del líquido (ver la figura 4). Tire del émbolo para cargar la dosis correcta en la jeringa (ver la figura 5).

Figura 4: Punta dentro del líquido | Figura 5: Extraiga la dosis correcta |
- Antes de sacar la aguja del vial, compruebe si hay burbujas de aire en la jeringa. Si hay burbujas de aire, sujete el vial y la jeringa con la aguja recta hacia arriba y golpee ligeramente en un lado de la jeringa hasta que las burbujas floten hacia arriba. Expulse las burbujas con el émbolo y extraiga líquido de nuevo hasta conseguir la dosis correcta (ver la figura 6).

Figura 6: Extraiga las burbujas de
aire y llene de nuevo la jeringa
- Saque la aguja del vial y sustituya la tapa protectora. No deje que la aguja toque nada. Ahora ya está listo para inyectar (ver la figura 7).

Figura 7: Listo
para inyectar
Inyección de la dosis:
Inyecte INCRELEX siguiendo las instrucciones del médico.
No administre la inyección si usted no va a poder comer un poco antes o después de la inyección.
- Escoja una zona para la inyección, ya sea el brazo, el muslo, una nalga o el abdomen (ver más abajo). Conviene cambiar el lugar de inyección en cada ocasión (rotar el lugar de inyección).
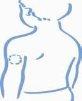
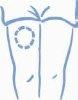

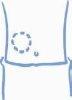
Brazo Muslo Nalga Abdomen
- Use alcohol o agua y jabón para limpiar la zona de la piel donde va a inyectar. El lugar de inyección debe estar seco antes de la inyección.
- Pellizque ligeramente la piel. Inserte la aguja como le enseñó el médico. Suelte la piel (ver la figura A).
Figura A: Pellizque ligeramente la piel e inyecte como le explicaron |
- Empuje despacio el émbolo de la jeringa hasta el final, procurando inyectar todo el líquido. Tire recto de la aguja para sacarla y presione suavemente el punto de inyección con una gasa o un algodón durante unos segundos. No frote el área(ver la figura B).

Figura B: Presione (no
frote) con gasa o algodón
- Siga las instrucciones del médico para deshacerse de la aguja y la jeringa. No le ponga la tapa de nuevo a la jeringa. La aguja y la jeringa usadas deben ponerse en un contenedor para objetos punzantes (por ejemplo, un contenedor para residuos biológicos peligrosos), un recipiente de plástico rígido (por ejemplo, una botella de detergente) o un recipiente de metal (como una lata de café vacía). Estos recipientes deben sellarse y desecharse de manera adecuada del modo que le explique su médico.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a INCRELEX 10 mg/ml SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 12 mg somatropinaPrincipio activo: SomatropinaFabricante: Pfizer S.L.Requiere recetaForma farmacéutica: INYECTABLE, 5,3 mg somatropinaPrincipio activo: SomatropinaFabricante: Pfizer S.L.Requiere recetaForma farmacéutica: INYECTABLE, 0,2 mg somatropinaPrincipio activo: SomatropinaFabricante: Pfizer S.L.Requiere receta
Médicos online para INCRELEX 10 mg/ml SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de INCRELEX 10 mg/ml SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes