
GREGAL 10 MICROGRAMOS/DOSIS LIBERADA POLVO PARA INHALACION (CAPSULA DURA)

Cómo usar GREGAL 10 MICROGRAMOS/DOSIS LIBERADA POLVO PARA INHALACION (CAPSULA DURA)
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para elpaciente
Gregal 10 microgramos/dosisliberadapolvo para inhalación (cápsula dura)
tiotropio
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted,y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Gregal y para qué se utiliza
- Qué necesita saber antes de empezar a usar Gregal
- Cómo usar Gregal
- Posibles efectos adversos
- Conservación de Gregal
- Contenido del envase e información adicional
1. Qué es Gregal y para qué se utiliza
Gregal contiene el principio activo tiotropio. Tiotropio ayuda a las personas con enfermedad pulmonar obstructiva crónica (EPOC) a respirar más fácilmente. La enfermedad pulmonar obstructiva crónica (EPOC) es una enfermedad pulmonar crónica que provoca dificultad para respirar y tos. El término EPOC está asociado con bronquitis crónica y enfisema. Dado que la EPOC es una enfermedad crónica usted debe usar este medicamento cada día y no sólo cuando tenga problemas respiratorios u otros síntomas de EPOC.
Gregal es un broncodilatador de acción prolongada que ayuda a abrir las vías respiratorias y facilita la toma y expulsión de aire de los pulmones. El uso regular de este medicamento también puede ayudarle cuando tiene dificultad para respirar continuada debida a su enfermedad y le ayudará a minimizar los efectos de la enfermedad en su vida diaria. También le ayuda a estar activo más tiempo. El uso diario de este medicamento también le ayudará a prevenir los síntomas repentinos y a corto plazo de empeoramiento de su EPOC, que pueden durar varios días. El efecto de este medicamento dura 24 horas, por lo tanto sólo necesita usarlo una vez al día.
Este medicamento no se debe utilizar como terapia de rescate para el tratamiento de la opresión en el pecho, tos, pitos o falta de respiración repentinos. Por favor, use un inhalador de acción rápida (rescate), como el salbutamol. Lleve consigo este inhalador de rescate en todo momento.
2. Qué necesita saber antes de empezar a usar Gregal
No use Gregal:
- si es alérgico a tiotropio o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si es alérgico a la atropina o medicamentos similares como ipratropio u oxitropio
- si es alérgico a la lactosa u otros azúcares
Advertencias y precauciones
Consulte a su médico,farmacéutico o enfermero antes de empezar a usar Gregal:
- si está tomando otros medicamentos que contengan ipratropio u oxitropio
- si sufre de glaucoma de ángulo estrecho, problemas de próstata o tiene dificultades para orinar
- si tiene algún problema renal
- si ha sufrido un infarto de miocardio en los últimos 6 meses, latidos del corazón irregulares inestables o que hayan puesto en peligro su vida, o fallo cardíaco grave en el pasado año.
Gregal está indicado para el tratamiento de mantenimiento de su enfermedad pulmonar obstructiva crónica, no debe utilizarse para tratar un episodio repentino de falta de respiración o pitos (sibilancias).
Tras la administración de Gregal pueden aparecer reacciones alérgicas inmediatas tales como erupción, hinchazón, picor, pitos o falta de respiración. Si esto ocurre, por favor consulte a su médico inmediatamente.
Los medicamentos inhalados como Gregal pueden provocar opresión en el pecho, tos, pitos o falta de respiración inmediatamente después de la inhalación. Si esto ocurre, use inmediatamente un inhalador de acción rápida (rescate), como el salbutamol. Si estos síntomas aparecen, interrumpa el uso de Gregal y consulte a su médico inmediatamente.
Tenga cuidado que el polvo para inhalación no entre en los ojos, ya que esto puede causar ojos llorosos, o podría provocar o empeorar un glaucoma de ángulo estrecho, que es una enfermedad de los ojos. El dolor o molestia ocular, visión borrosa, halos visuales o imágenes coloreadas asociados con enrojecimiento de los ojos pueden ser signos de un episodio agudo de glaucoma de ángulo estrecho. Los síntomas oculares pueden ir acompañados de dolor de cabeza, náuseas o vómitos. Debe interrumpir el uso de este medicamento y consultar inmediatamente a su médico, preferiblemente un oftalmólogo, cuando aparezcan los signos y síntomas de glaucoma de ángulo estrecho.
Los medicamentos inhalados pueden disminuir la secrección normal (saliva) en su boca y producir sequedad de boca. Puede asociarse a largo plazo con caries dental. Por lo tanto, acuérdese de cuidar su higiene bucal, enjuague su boca y cepille sus dientes regularmente.
En caso de que haya sufrido un infarto de miocardio en los últimos 6 meses, latidos del corazón irregulares inestables o que hayan puesto en peligro su vida, o fallo cardíaco grave en el pasado año, informe a su médico. Esto es importante para decidir si Gregal es el medicamento adecuado para usted.
No debe usar este medicamento más de una vez al día (ver sección 3).
Niños y adolescentes
Gregal no está recomendado para niños y adolescentes menores de 18 años.
Uso de Gregal conotros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento, incluyendo cualquier otro inhalador y medicamentos adquiridos sin receta.
Informe a su médico o farmacéutico si está utilizando o ha utilizado medicamentos similares para su enfermedad pulmonar, tales como ipratropio u oxitropio.
No se ha informado de reacciones adversas específicas cuando este medicamento ha sido utilizado junto a otros medicamentos utilizados habitualmente para el tratamiento de la EPOC como los inhaladores de rescate, por ej. salbutamol, metilxantinas, como la teofilina y/o esteroides orales e inhalados, como por ejemplo la prednisolona.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento. No debe utilizar este medicamento a menos que específicamente se lo haya recomendado su médico.
Conducción y uso de máquinas
La aparición de mareos, visión borrosa o dolor de cabeza pueden influir en la capacidad para conducir y utilizar máquinas.
Gregal contiene lactosa
Lactosa es un tipo de azúcar encontrado en la leche de vaca. Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de usar este medicamento. Puede provocar reacciones alérgicas en pacientes con alergia a la proteína de la leche de vaca.
Si se administra según la dosis recomendada, una cápsula una vez al día, cada dosis proporciona hasta 18 mg de lactosa monohidrato.
3. Cómo usar Gregal
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es la inhalación del contenido de unacápsula una vez al díacon el inhalador Zonda. Una cápsula proporciona la dosis diaria necesaria de tiotropio (dosis liberada de 10 microgramos de tiotropio). No use más de la dosis recomendada.
Debe intentar usar este medicamento a la misma hora cada día. Esto es importante ya que este medicamento es eficaz durante 24 horas.
Las cápsulas son para inhalación exclusivamente y no para la ingesta oral.
No ingerir las cápsulas.
El inhalador Zonda, en el que usted debe introducir la cápsula de Gregal, perfora la cápsula y le permite aspirar el polvo. Las cápsulas sólo deben ser inhaladas utilizando el inhalador Zonda. No use otros inhaladores para administrar las cápsulas de Gregal.
Asegúrese que sabe cómo usar el inhalador Zonda correctamente. Las instrucciones de uso del inhalador se encuentran detrás de este prospecto. Recuerde seguir detenidamente estas instrucciones de uso. Además se proporcionan imágenes adicionales sobre la correcta colocación de la cápsula en el inhalador en el interior de la tapa del envase. Para evitar el riesgo de asfixia, NUNCAcoloque una cápsula directamente dentro de la boquilla.Si tiene algún problema para utilizar el inhalador Zonda, pregúntele a su médico, enfermera o farmacéutico para que le enseñe cómo funciona.
Si es necesario, puede limpiar la boquilla de su inhalador Zonda tras su uso con un pañuelo de papel seco.
Asegúrese de que no sopla dentro del inhalador Zonda. Cuando use Gregal, tenga cuidado y no deje que entre el polvo en sus ojos. Si le entrara polvo en los ojos podría provocarle visión borrosa, dolor y/o enrojecimiento ocular. Debe lavarse los ojos inmediatamentecon agua templada. Consulte a su médico inmediatamentepara más información.
Si nota que su respiración empeora, consulte a su médico tan pronto como sea posible.
Uso en niños y adolescentes
Gregal no está recomendado para niños y adolescentes menores de 18 años.
Si usa más Gregal del que debe
Si usted inhala más de 1 cápsula de Gregal en un día, debe hablar con su médico inmediatamente. Puede tener mayor riesgo de sufrir una reacción adversa como sequedad de boca, estreñimiento, dificultad para orinar, aumento del ritmo cardíaco o visión borrosa.
Si olvidó usar Gregal
Si usted ha olvidado administrarse una dosis, hágalo tan pronto como lo recuerde, pero nodos dosis a la vez o en el mismo día. Luego adminístrese su próxima dosis como siempre. No administre una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Gregal
Antes de interrumpir el tratamiento con Gregal debe hablar con su médico o farmacéutico. Si interrumpe el uso de este medicamento, los signos y síntomas de EPOC pueden empeorar.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Tras el uso de este medicamento pueden producirse de forma individual o como parte de una reacción alérgica grave (reacción anafiláctica) reacciones adversas graves que incluyen reacciones alérgicas que causan tumefacción de la cara y la garganta (angioedema) u otras reacciones de hipersensibilidad (tales como disminución repentina de su presión arterial o mareo), o aumento de las sibilancias y falta de aire. Adicionalmente, como ocurre con todos los medicamentos inhalados, algunos pacientes pueden experimentar presión inesperada en el pecho, tos, sibilancias o dificultad para respirar inmediatamente después de la inhalación (broncoespasmo).
Si le ocurre cualquiera de estas reacciones consulte a su médico inmediatamente.
No use Gregal de nuevo hasta que vea o al menos hable con su médico. Si tiene sibilancias y falta de aliento use su inhalador de acción rápida (de rescate) inmediatamente.
Otros efectos adversos han sido experimentados por personas que han usado este medicamento y se listan de acuerdo con su frecuencia:
Frecuentes: pueden afectar hasta 1 de cada 10 personas
- Sequedad de boca: ésta es generalmente leve
Poco frecuentes: pueden afectar hasta 1 de cada 100 personas
- mareos
- dolor de cabeza
- alteraciones del gusto
- visión borrosa
- ritmo cardíaco irregular (fibrilación auricular)
- inflamación de la garganta (faringitis)
- ronquera (disfonía)
- tos
- ardor de estómago (reflujo gastroesofágico)
- estreñimiento
- infección fúngica en la cavidad oral o la garganta (candidiasis orofaríngea)
- erupción
- dificultad para orinar (retención urinaria)
- dolor al orinar (disuria)
Raras: pueden afectar hasta 1 de cada 1.000 personas
- dificultad para dormir (insomnio)
- halos visuales o imágenes coloreadas asociados con enrojecimiento de los ojos (glaucoma)
- aumento de la presión ocular
- ritmo cardíaco irregular (taquicardia supraventricular)
- aumento del ritmo cardíaco (taquicardia)
- palpitaciones
- presión en el pecho, asociado con tos, sibilancias o dificultad para respirar inmediatamente después de la inhalación (broncoespasmo)
- hemorragia nasal (epistaxis)
- inflamación de la laringe (laringitis)
- inflamación de los senos paranasales (sinusitis)
- bloqueo intestinal o ausencia de movimiento del intestino (obstrucción intestinal incluyendo íleo paralítico)
- inflamación de las encías (gingivitis)
- inflamación de la lengua (glositis)
- dificultad al tragar (disfagia)
- inflamación de la boca (estomatitis)
- sensación de mareo (náuseas)
- hipersensibilidad, incluyendo reacciones inmediatas
- reacción alérgica grave que causa tumefacción de la cara y la garganta (angioedema)
- urticaria
- picor (prurito)
- infección del tracto urinario
No conocida: no puede estimarse a partir de los datos disponibles
- pérdida de agua corporal (deshidratación)
- caries dental
- infecciones o ulceraciones de la piel
- sequedad de la piel
- tumefacción de las articulaciones
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Gregal
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y etiqueta del frasco después de CAD o EXP. La fecha de caducidad es el último día del mes que se indica.
No refrigerar ni congelar.
Mantener el frasco perfectamente cerrado. Conservar en el embalaje original para protegerlo de la humedad.
Utilice este medicamento durante los 30 días (frasco de 15 cápsulas) o 60 días (frasco de 30 cápsulas) tras la apertura del frasco.
El inhalador Zonda sólo se puede usar con el frasco de cápsulas que se proporciona. No reutilice el inhalador para otro frasco de cápsulas.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesite en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
El dispositivo inhalador debe desecharse de acuerdo con los requisitos locales.
6. Contenido del envase e información adicional
Composición de Gregal 10 microgramos/dosisliberadapolvo para inhalación
- El principio activo es tiotropio. Cada cápsula contiene 13 microgramos de principio activo tiotropio (como bromuro de tiotropio). Durante la inhalación se liberan 10 microgramos de tiotropio por cada cápsula desde la boquilla del inhalador Zonda y son inhalados hasta los pulmones.
- Los demás componentes son lactosa monohidrato (contenido de la cápsula) e hidromelosa (cubierta de la cápsula).
Aspecto del producto y contenido del envase
Gregal 10 microgramos/dosis liberada polvo para inhalación es una cápsula dura incolora y transparente que contiene polvo de color blanco.
Este medicamento se presenta en frascos, con cierre con tapón de rosca. El frasco se presenta en una caja con el inhalador Zonda. El inhalador Zonda tiene un cuerpo y una tapa de color verde con un pulsador de color blanco.
Gregal está disponible en envases con 15 cápsulas o 30 cápsulas y un inhalador Zonda.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Teva Pharma, S.L.U.
C/Anabel Segura, 11 Edificio Albatros B, 1ª Planta
Alcobendas, 28108 Madrid (España)
Responsable de la fabricación
Laboratorios LICONSA S.A.
Avda. Miralcampo, No 7, Polígono Industrial Miralcampo
19200 Azuqueca de Henares, Guadalajara
España
o
Actavis Ltd
BLB015, BLB016, Bulebel Industrial Estate,
Zejtun, ZTN3000,
Malta
Representante local:
Laboratorios BIAL, S.A.
C/ Alcalá 265, Edificio 2, Planta 2
28027 Madrid España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
España: Gregal 10 microgramos/dosis liberada polvo para inhalación
Portugal: Gregal
Fecha de la última revisión de esteprospecto: Febrero 2021
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
<-----------------------------------------------------------------------------------------------------------------?
Instrucciones de uso de Zonda
Estimado paciente,
El inhalador Zonda le permite inhalar el medicamento contenido en la cápsula de Gregal que su médico le ha prescrito para sus problemas respiratorios.
Recuerde seguir cuidadosamente las instrucciones de su médico para utilizar Gregal. El inhalador Zonda está especialmente diseñado para las cápsulas de Gregal, por lo que no se debe utilizar para otro medicamento.
Las cápsulas sólo se deben inhalar usando el inhalador Zonda. Noutilice otros inhaladores para administrar las cápsulas de Gregal. Cada cápsula contiene sólo una pequeña cantidad de polvo. Noabra la cápsula o podría no funcionar.
El inhalador Zonda se debe utilizar con el frasco de cápsulas que se proporciona. No reutilice el inhalador para cualquier otro frasco de cápsulas. Deseche el dispositivo Zonda tras los 15 usos (si ha usado el frasco de 15 cápsulas) o 30 usos (si ha usado el frasco de 30 cápsulas).
Zonda

- Capuchón protector
- Boquilla
- Base
- Botón perforador
- Cámara central
- Tire del capuchón protector hacia arriba

- Mantenga la base del inhalador firmemente. Después abrir la boquilla levantándola hacia arriba, en la dirección de la flecha.

- Extraer una cápsula de Gregal del frasco inmediatamente antes de su uso y cierre el frasco perfectamente. Coloque una cápsula en la cámara central en la base del inhalador. No guarde la cápsula en el inhalador Zonda.
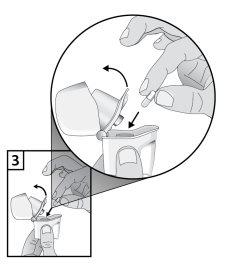
- Para evitar el riesgo de asfixia, NUNCA coloque una cápsula directamente dentro de la boquilla.
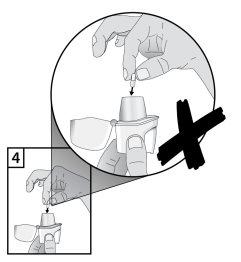
- Cerrar la boquilla firmemente hasta oír un clic, dejando abierto el capuchón protector.
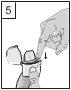
- Coger el inhalador con la boquilla hacia arriba, presionar a fondo el botón perforador una vez y soltarlo. Esta maniobra perfora la cápsula y permite que se libere el medicamento cuando se aspira.
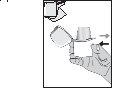
- Espirar a fondo. Importante: nunca se debe espirar dentro de la boquilla.

- Llevar el inhalador a la boca y mantener la cabeza en posición derecha. Cerrar los labios fuertemente alrededor de la boquilla y aspirar lenta y profundamente pero de forma suficiente como para oír o notar vibrar la cápsula dentro de la cámara central. Aguante la respiración durante el tiempo que se sienta cómodo mientras saca el inhalador de su boca. Continuar respirando normalmente. Repetir los pasos 7 y 8 una vez más; esto vaciará la cápsula completamente.
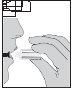
- Tras el uso, abrir la boquilla otra vez y tirar la cápsula vacía. Cerrar la boquilla y el capuchón protector para guardar el inhalador Zonda.
El inhalador Zonda es un Dispositivo Médico (CE).
Fabricante:
Laboratorios Liconsa S.A.
Poligono Industrial Miralcampo
Avda. Miralcampo, 7
19200 Azuqueca de Henares – Guadalajara, España

0051
- País de registro
- Precio medio en farmacia39.25 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a GREGAL 10 MICROGRAMOS/DOSIS LIBERADA POLVO PARA INHALACION (CAPSULA DURA)Forma farmacéutica: INHALACIÓN PULMONAR, 10 MICROGRAMOSPrincipio activo: tiotropium bromideFabricante: Teva Pharma S.L.U.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 18 microgramosPrincipio activo: tiotropium bromideFabricante: Boehringer Ingelheim International GmbhRequiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 2,5 microgramos / pulverizaciónPrincipio activo: tiotropium bromideFabricante: Boehringer Ingelheim International GmbhRequiere receta
Médicos online para GREGAL 10 MICROGRAMOS/DOSIS LIBERADA POLVO PARA INHALACION (CAPSULA DURA)
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de GREGAL 10 MICROGRAMOS/DOSIS LIBERADA POLVO PARA INHALACION (CAPSULA DURA), sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















