
FRAGMIN 2,500 IU/mL Injectable Solution in Vials

How to use FRAGMIN 2,500 IU/mL Injectable Solution in Vials
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
FRAGMIN2,500 IU/mlinjectable solution in vials
dalteparin sodium
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Fragmin and what is it used for
- What you need to know before you use Fragmin
- How to use Fragmin
- Possible side effects
- Storage of Fragmin
- Contents of the pack and further information
1. What is Fragmin and what is it used for
Fragmin belongs to a group of medicines called low molecular weight heparins.
Fragmin 2,500 IU/ml is used in children and adolescents from 1 month of age for the treatment of blood clots in the veins (venous thromboembolism or VTE).
2. What you need to know before you use Fragmin
Do not use Fragmin if:
- you are allergic (hypersensitive) to dalteparin sodium or any of the other ingredients of this medicine (listed in section 6), to any other type of heparin (medicines that prevent blood clotting) other than dalteparin sodium, or to products derived from pigs,
- you have acute gastroduodenal ulcer, cerebral hemorrhage, or other significant bleeding,
- you have severe coagulation disorders,
- you have a disease called acute or subacute septic endocarditis (inflammation of one of the heart membranes due to infection),
- you have had an operation on the central nervous system, eyes, or ears, or if you have injuries to these organs or systems,
- you have a decrease in the number of platelets (cells present in the blood that are involved in blood clotting) and when a test of aggregation in the presence of dalteparin sodium is performed, the result is positive.
If you are being treated with Fragmin, you will not be able to receive epidural or spinal anesthesia.
Warnings and precautions:
Consult your doctor, pharmacist, or nurse before starting to use Fragmin if:
- You are going to receive intramuscular injections of other medicines due to the risk of hematomas.
- You have a decrease in the number of platelets or defects in them. Your doctor may request specific tests to determine the cause of this problem.
- You have severe renal impairment (decreased kidney function) or severe hepatic impairment (decreased liver function).
- You have uncontrolled high blood pressure.
- You have retinal disorders (a part of the eye) due to diabetes or high blood pressure.
- You have recently undergone surgery or have a high risk of bleeding.
- You have had an acute myocardial infarction and are being treated with this medicine.
- You are at risk of elevated potassium levels in the blood due to some disease or due to taking certain medications. Your doctor may perform tests to measure potassium levels.
- You are undergoing epidural (in one of the membranes surrounding the brain and spinal cord) or spinal anesthesia (in the spinal cord), or a lumbar puncture, and you are being administered heparin for preventive purposes; very rarely, hematomas may occur in these areas. If you feel back pain, numbness, weakness in the lower limbs, or any disorder in the functioning of the intestine or bladder, inform your doctor immediately.
- You have a cardiac valve prosthesis; the preventive doses of Fragmin may not be sufficient to prevent valve thrombosis.
- You are receiving prolonged treatment for unstable coronary disease, such as prior to revascularization; your doctor may reduce your dose of Fragmin.
Considering your condition and/or age, your doctor may perform tests to control anticoagulant activity and avoid the risk of bleeding or recurrence of thrombosis.
Fragmin should not be exchanged with other unfractionated heparins, low molecular weight heparins, or synthetic polysaccharides, as their effect may not be the same.
Children and adolescents:
Fragmin is not used for babies under 1 month of age.
Other medicines and Fragmin:
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those obtained without a prescription.
Certain medicines may interact with Fragmin; in these cases, it may be convenient to change the dose or interrupt treatment with one of the medicines.
Thrombolytic treatment (which dissolves the clot) or some medicines that affect blood clotting may increase the risk of bleeding when combined with Fragmin:
- Aspirin (acetylsalicylic acid).
- Platelet inhibitors (used to decrease platelet aggregation and reduce the risk of blood clots).
- Thrombolytics (used to dissolve blood clots).
- Non-steroidal anti-inflammatory drugs (NSAIDs) (medicines used to treat inflammation).
- Vitamin K antagonists and other types of anticoagulants.
- Dextran (used in some artificial tears).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Consult your doctor or pharmacist before taking or using a medicine, including Fragmin.
If you are pregnant, you should only use this medicine when clearly necessary, although Fragmin does not cross the placenta.
It is not recommended to use it with epidural anesthesia. Inform your doctor if you have an artificial heart valve.
If you are breastfeeding, inform your doctor; he will assess whether treatment with this medicine is appropriate, as Fragmin passes into breast milk in small amounts.
Driving and using machines:
This medicine does not alter the ability to drive vehicles or use machinery.
Fragmin contains sodium:
This medicine contains 44.1 mg of sodium (main component of table salt/cooking salt) per vial. This is equivalent to 2.21% of the maximum recommended daily intake of sodium for an adult.
.
3. How to use Fragmin
Follow exactly the administration instructions of this medicine indicated by your doctor. Consult your doctor, pharmacist, or nurse if you have doubts.
Remember to use your medicine.
Your doctor will indicate the dose, method of use, and duration of your treatment with Fragmin.
Use in children and adolescents:
Treatment of blood clots in the veins (symptomatic venous thromboembolism [VTE])
The recommended doses depend on the child's body weight and age group. The doctor will perform the calculation. The doctor will advise on the individualized dose of Fragmin according to these criteria. Do not change the dose or treatment schedule without consulting your doctor first.
The following table shows the recommended initial dose for children and adolescents based on their age:
Children from 1 month to less than 2 years:150 IU/kg twice a day.
Children from 2 years to less than 8 years:125 IU/kg twice a day.
Children from 8 years to less than 18 years:100 IU/kg twice a day.
The effect of Fragmin will be controlled after the initial dose and subsequent dose adjustment through a blood test.
How to inject Fragmin
Your medicine will be administered generally by a doctor or nurse. Fragmin is administered under the skin (subcutaneously).
In children, Fragmin is usually injected into a skin fold in the abdomen (area in the shape of a "U" around the navel) or in the central area of the thighs.
On occasion, you may need to receive injections of Fragmin outside the hospital setting. If dilution is necessary before administering Fragmin to children, it must be performed by a healthcare professional. You must follow your doctor's instructions on how and when to inject the diluted medicine provided (see the section "Follow the steps explained below").
This section of the leaflet explains how to inject Fragmin yourself or to your child. You must follow these instructions only after being trained by your doctor. If you are unsure of what to do, talk to your doctor immediately. The dose of Fragmin should be injected (or administered) at the time recommended by your doctor.
Follow the steps explained below
Step 1: preparing the syringe for injection:
Gather all the materials you need: vial, syringe, cotton with alcohol or water and soap. The vial, syringe, and needle have protective caps. The vial's flip-top cap can rotate; this is normal. Check that all caps are properly placed, and if they are not, do not use them. If a needle is bent, do not use it.
Before starting, make sure you know how much to inject. Your doctor should have indicated the correct amount of solution to administer. If the doctor has not given you this instruction, contact him/her.
Prepare the dose of the medicine:remove the plastic cap from the top of the vial (if present). Do not remove the rubber stopper or the aluminum ring around the top of the vial. Clean the rubber stopper of the vial with an alcohol swab. After cleaning, do not touch the stopper with your hands or allow it to touch any surface (see Figures 1 and 2).
Figure 1 Figure 2
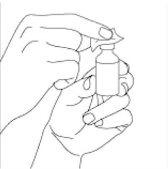
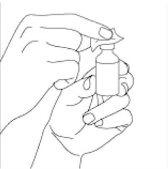
Extract the correct dose from the vial:Remove the syringe from the plastic or paper cover. Remove the cap that covers the needle. Be careful not to touch the needle. With the vial in a vertical position, push the needle down at a 90-degree angle in the vial stopper. Be careful not to bend the needle (see Figure 3).
Figure 3
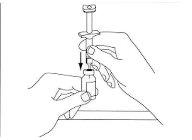
Put the vial upside down, keeping the needle attached to the syringe in the vial. The needle and syringe will point upwards (see Figure 4).
Figure 4
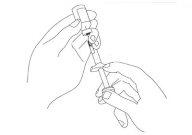
Make sure the tip of the needle is completely covered by the medicine. Pull the syringe plunger back to the correct dose, checking the dose level marks on the side of the syringe cylinder (see Figure 5).
Figure 5
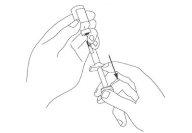
Keep the vial upside down, with the needle in the vial pointing upwards. Gently tap the syringe or "move" it with your fingertips. This helps to displace the bubbles to the top of the syringe (see Figure 6).
Figure 6
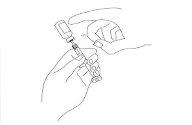
Once the bubbles are at the top of the syringe, gently push the plunger to force the bubbles out of the syringe and back into the vial. Slowly pull the syringe plunger back to the correct dose, avoiding bubbles.
After removing the bubbles, check the amount of medicine in the syringe according to the dose marks on the side of the syringe cylinder to ensure it is correct.
Now you are ready to inject. Continue with Step 2.
Step 2: choosing and preparing the subcutaneous injection site
If you are injecting yourself or an adult, you can inject Fragmin into an area on the right or left side of your stomach or towards either side (see the shaded areas in Figure 7).
Figure 7
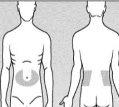
If you are injecting a child, choose one of the recommended injection sites below (see the shaded areas in Figure 8):
A "U"-shaped area around the navel.
Central area of the thighs.
Figure 8
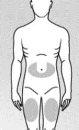
- Use a different injection site each time you administer a dose.
- Do not administer the injection in areas where the skin is painful, bruised, red, or hard. Avoid areas with scars.
- If you or the child have psoriasis, do not administer the injection directly into any raised, thick, red, or scaly skin lesions ("psoriatic skin lesions").
- Wash and dry your hands.
- Clean the injection site with a new alcohol swab, using circular motions. Let the skin dry completely. Do not touch this area again before administering the injection.
Step 3: adopting the correct position
You or your child should be sitting or lying down for the subcutaneous injection. If you are going to self-inject the medicine, sit in a comfortable position where you can see your abdomen (see Figure 9).
Figure 9
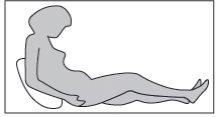
Step 4:
With your thumb and index finger, lift a skin fold with one hand. With the other hand, hold the syringe like a pencil. This will be the injection site.
Step 5:
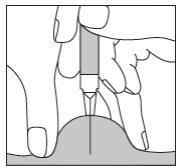
If you are injecting Fragmin to an adult or yourself,hold the syringe over the skin fold, keeping it at a right angle (i.e., vertically, as in the diagram, and not at an angle). Insert the needle into the skin until it is completely inside (see Figure 10).
Figure 10
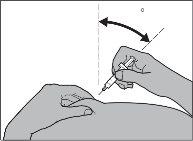
If you are going to inject Fragmin to a child,insert the needle into the skin until the end, with a small, quick movement, at an angle of 45° to 90° (see Figure 11).
Figure 11
Step 6:
Step 6:
Push the plunger completely down at a slow and constant speed to administer the correct dose. Continue to pinch the skin fold while administering the injection, and then release the skin fold and remove the needle.
If there is any bleeding at the injection site, apply gentle pressure. Do not rub the injection site, as this may cause hematomas.
Press the injection site with a cotton swab for 10 seconds. A slight bleeding may occur. Do not rub the injection site. You can place a bandage over the injection site.
Step 7:
Discard the syringe and needle in a sharps container. Keep your sharps container out of the reach of others. When the sharps container is almost full, discard it according to the instructions or talk to your doctor or nurse.
If you use more Fragmin than you should
If you have used more Fragmin than you should, consult your doctor or pharmacist immediately, go to the nearest hospital, or contact the Toxicology Information Service, phone 91 562 04 20.
If you forget to use Fragmin
Consult your doctor or pharmacist immediately.
Do not administer a double dose to make up for forgotten doses.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible Adverse Effects
Like all medicines, Fragmin can cause adverse effects, although not all people suffer from them.
Frequently observed adverse effects (may affect up to one in 10 patients):
- Pain and bruising at the injection site
- Reversible decrease in the number of blood platelets not mediated by immune mechanisms (type 1)
- Bleeding at any site, which has sometimes been fatal
- Temporary increase in liver enzymes
Rare adverse effects (may affect up to one in 1,000 patients):
- Hair loss, skin cell death
Adverse effects of unknown frequency (cannot be estimated from available data):
- Decrease in the number of blood platelets mediated by immune mechanisms induced by heparin (type 2)
- Severe allergic reactions
- Localized bleeding inside the skull, inside the abdomen, or in other locations, sometimes fatal
- Rash
- Accumulation of blood inside the skull or spinal column (epidural or spinal hematoma)
- Elevation of blood potassium levels
- Osteoporosis (porous bones)
It is expected that adverse effects in children will be the same as those in adults. However, there is limited information on possible adverse effects with prolonged use in children.
Reporting of Adverse Effects:
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Fragmin
Keep out of sight and reach of children.
Do not store at a temperature above 25°C
Do not use Fragmin after the expiration date (CAD) shown on the packaging and outer carton. The expiration date is the last day of the indicated month.
Do not use Fragmin if you observe visible signs of deterioration.
From a microbiological point of view, the product should be used immediately. If not used immediately, the storage times and conditions are the responsibility of the user.
Medicines should not be thrown away in drains or trash. Deposit the packaging and medicines you no longer need at the SIGRE Point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Fragmin 2,500 IU/ml:
The active ingredient of Fragmin 2,500 IU/ml is dalteparin sodium.
Each milliliter of solution contains 2,500 IU (anti-Xa) of dalteparin sodium. The total content per 4 ml vial is 10,000 IU (anti-Xa) (= 10,000 IU/4 ml).
The other components are: sodium chloride, sodium hydroxide, hydrochloric acid, and water for injectable preparations.
Appearance of Fragmin 2,500 IU/ml and Package Contents:
Vials of 4 ml: injectable solution for subcutaneous administration in vials with 10,000 IU (anti-Xa)/4 ml (2,500 IU/ml).
Marketing Authorization Holder:
Pfizer, S.L.
Avda. de Europa 20-B.
Parque Empresarial La Moraleja
28108 Alcobendas (Madrid)
Manufacturer:
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870 Puurs-Sint-Amands
Belgium
Additional Information for Healthcare Professionals:
Fragmin 2,500 IU/ml can be diluted with sodium chloride infusion solutions (9 mg/ml) or glucose (50 mg/ml) in glass bottles and plastic containers.
.
The compatibility of this medicine with other products has not been investigated except for those mentioned above.
Date of the Last Revision of this Prospectus: June 2025
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Average pharmacy price71.65 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FRAGMIN 2,500 IU/mL Injectable Solution in VialsDosage form: INJECTABLE, 10,000 IU dalteparin sodium/0.4 mlActive substance: dalteparinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 10,000 IU/mlActive substance: dalteparinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 12,500 IU dalteparin sodium/0.5 mlActive substance: dalteparinManufacturer: Pfizer S.L.Prescription required
Online doctors for FRAGMIN 2,500 IU/mL Injectable Solution in Vials
Discuss questions about FRAGMIN 2,500 IU/mL Injectable Solution in Vials, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















