
ФОСКАРНЕТ КАБИ 24 мг/мл РАСТВОР ДЛЯ ИНФУЗИЙ
Спросите врача о рецепте на ФОСКАРНЕТ КАБИ 24 мг/мл РАСТВОР ДЛЯ ИНФУЗИЙ

Инструкция по применению ФОСКАРНЕТ КАБИ 24 мг/мл РАСТВОР ДЛЯ ИНФУЗИЙ
Введение
Проспект: информация для пользователя
Фоскарнет Кabi 24 мг/мл раствор для инфузии ЕФГ
фоскарнет натрия гексагидрат
Прочитайте внимательно весь проспект перед началом использования этого лекарства, поскольку он содержит важную информацию для вас.
- Сохраните этот проспект, поскольку вам может потребоваться прочитать его снова.
- Если у вас есть какие-либо сомнения, проконсультируйтесь с вашим врачом или медсестрой.
- Это лекарство было назначено только вам, и его не следует давать другим людям, даже если они имеют те же симптомы, что и вы, поскольку оно может нанести им вред.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом или медсестрой, даже если это побочные эффекты, которые не указаны в этом проспекте. См. раздел 4.
Содержание проспекта
- Что такое Фоскарнет Кabi и для чего он используется
- Что вам нужно знать перед началом использования Фоскарнет Кabi
- Как использовать Фоскарнет Кabi
- Возможные побочные эффекты
- Хранение Фоскарнет Кabi
- Содержание упаковки и дополнительная информация
1. Что такое Фоскарнет Кabi и для чего он используется
Это лекарство относится к группе лекарств, называемых противовирусными. Он действует на определенные вирусы [все вирусы, принадлежащие к группе герпеса и некоторые ретровирусы, такие как цитомегаловирус (ЦМВ)]. Он предотвращает размножение вируса в инфицированных клетках.
Фоскарнет используется у людей с ослабленной иммунной системой:
- Для лечения ретинита, вызванного ЦМВ, у пациентов с СИДой.
- Для лечения герпетической инфекции (на губах, носу, глазах и гениталиях).
- Фоскарнет вводится людям с инфекцией ЦМВ после трансплантации костного мозга. Это также известно как трансплантация стволовых клеток гемопоэза (ГСТ, по английски). В некоторых случаях инфекция может быть обнаружена до того, как пациент начнет проявлять симптомы. Это называется виремией ЦМВ.
2. Что вам нужно знать перед началом использования Фоскарнет Кabi
Не используйте Фоскарнет Кabi:
- Если вы аллергичны к фоскарнету или к любому другому компоненту этого лекарства (указанному в разделе 6).
ПРОКОНСУЛЬТИРУЙТЕСЬ С ВАШИМ ВРАЧОМ ИЛИ ФАРМАЦЕВТОМ, ЕСЛИ ВЫ НЕ УВЕРЕНЫ.
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом или медсестрой перед началом использования этого лекарства:
- Если у вас есть проблемы с почками. В этом случае ваш врач может скорректировать дозу в зависимости от вашего состояния.
- Если у вас низкий уровень кальция в крови до начала лечения этим лекарством и если вы принимаете лекарство, снижающее уровень кальция в крови.
- Если у вас есть проблемы с сердцем.
- Если у вас есть онемение в руках или губах и тошнота. В этом случае ваша медсестра уменьшит скорость инфузии.
- В случае случайного контакта с препаратом, промойте пораженную кожу или слизистые оболочки (полость рта, носа, ушей или гениталий) водой.
Рекомендуется обратить особое внимание на личную гигиену после мочеиспускания: мытье полового члена (или вульвы) проточной водой для предотвращения генитальных повреждений (язв гениталий),
Анализ крови
До и во время лечения ваш врач может назначить вам анализ крови и мочи. Эти анализы направлены на проверку функции почек и уровня минералов в крови.
Другие лекарства и Фоскарнет Кabi
Сообщите вашему врачу или медсестре, если вы используете, недавно использовали или можете использовать любое другое лекарство, включая лекарства, приобретенные без рецепта, и травяные средства. Это связано с тем, что фоскарнет может влиять на действие некоторых лекарств, и некоторые лекарства могут влиять на фоскарнет.
В частности, сообщите вашему врачу или медсестре, если вы принимаете любое из следующих лекарств:
- Пентамидин (для инфекций).
- Амфотерицин В (для грибковых инфекций).
- Ацикловир (для вирусных инфекций).
- Антибиотики, называемые аминогликозидами, такие как гентамицин или стрептомицин (для инфекций).
- Циклоспорин А, метотрексат или такролимус (используемые для подавления иммунной системы).
- Лекарства, ингибирующие протеазу, такие как ритонавир и саквинавир.
- Хинидин, амиодарон, соталол или другие лекарства, которые могут влиять на ваш сердечный ритм.
- Транквилизаторы (нейролептики).
Беременность и лактация
Если вы беременны или кормите грудью, считаете, что можете быть беременной или планируете стать беременной, проконсультируйтесь с вашим врачом или фармацевтом перед использованием этого лекарства.
- Не рекомендуется использовать фоскарнет во время беременности.
- Вам следует избегать беременности во время лечения фоскарнетом и, поэтому, вам следует использовать эффективные методы контрацепции во время лечения фоскарнетом и в течение 7 месяцев после окончания лечения.
- Рекомендуется мужчинам, лечившимся фоскарнетом, использовать эффективные методы контрацепции и не должно быть зачатия во время или до 4 месяцев после лечения.
- Не принимайте фоскарнет во время лактации.
Вождение и использование машин
Фоскарнет может влиять на вашу способность управлять транспортными средствами или работать с машинами. Это связано с риском судорог, аномальных движений и головокружения, сообщаемых при использовании этого лекарства. Проконсультируйтесь с вашим врачом перед выполнением любой из этих действий.
Фоскарнет Кabi содержит натрий
Это лекарство содержит 240 мкмоль (5,5 мг) натрия (основного компонента столовой соли) в каждом мл. Это должно быть учтено пациентами, следующими диете с контролем натрия, поэтому мы просим вас сообщить вашему врачу или медсестре, если вы придерживаетесь диеты с низким содержанием натрия.
Максимальная рекомендуемая доза этого лекарства содержит 2,89 г натрия (присутствующего в столовой соли).
Это эквивалентно 144,5% максимальной суточной нормы потребления натрия, рекомендуемой для взрослых.
Проконсультируйтесь с вашим врачом или фармацевтом, если вам необходимо принимать фоскарнет ежедневно в течение длительного периода, особенно если вам рекомендована диета с низким содержанием соли.
3. Как использовать Фоскарнет Кabi
Следуйте точно инструкциям по введению этого лекарства, указанным вашим врачом. В случае сомнений проконсультируйтесь с вашим врачом снова.
- Фоскарнет будет введен вам врачом или медсестрой. Он будет введен в виде инфузии (капельницы) внутривенно. Он может быть введен через центральную вену в вашей груди, если у вас уже есть установленная вена в этом месте.
- Каждая инфузия будет длиться не менее 1 часа. Не вмешивайтесь в процесс инфузии во время введения.
- Доза фоскарнета, которую вам будет введена, будет зависеть от вашего веса и функции почек.
- Важно поддерживать адекватный прием жидкости во время инфузии, что также поможет предотвратить проблемы с почками. Если необходимо, ваш врач или медсестра введут вам режим гидратации одновременно с инфузией фоскарнета.
Личная гигиена
Мойте гениталии тщательно после мочеиспускания. Это поможет предотвратить развитие язв.
Если раствор фоскарнета попадет на кожу или глаза
Если по ошибке раствор фоскарнета попадет на кожу или глаза, промойте пораженную область водой сразу же.
Если вы примете больше Фоскарнет Кabi, чем следует
Если вам будет введена чрезвычайно высокая доза фоскарнета, сообщите об этом вашему врачу или фармацевту сразу же.
Если вы считаете, что вам было введено слишком много фоскарнета, сообщите об этом вашему врачу сразу же.
В случае передозировки проконсультируйтесь с вашим врачом или фармацевтом или позвоните в Центр токсикологической информации, телефон: 91 562 04 20, указав лекарство и количество, введенное.
4. Возможные побочные эффекты
Как и все лекарства, это лекарство может вызывать побочные эффекты, хотя не все люди испытывают их.
Некоторые побочные эффекты могут быть тяжелыми и требовать немедленного медицинского внимания:
- Тяжелые аллергические реакции, включая снижение артериального давления, шок и отек кожи (ангиоэдема). Они известны как гиперчувствительность, анафилактические или анафилактоидные реакции.
- Тяжелые кожные высыпания. Этот тип высыпаний может быть связан с покраснением, отеком и пузырями на коже, во рту, горле, глазах и других местах внутри организма и, в некоторых случаях, может привести к смерти. Они называются эритема мультиформе, синдром Стивенса-Джонсона и токсическая эпидермальная некролиз.
Если вы испытываете любой из вышеуказанных побочных эффектов, сообщите об этом вашему врачу сразу же или обратитесь в ближайшее отделение неотложной помощи.
Другие побочные эффекты включают:
Очень часто: могут возникать у более 1 из 10 человек
- Снижение количества красных кровяных клеток (показано в анализах). Вы можете чувствовать усталость или иметь бледный вид.
- Низкий уровень белых кровяных клеток (гранулоцитопения). Симптомы включают инфекции и высокую температуру (лихорадку).
- Снижение уровня калия в крови.
- Снижение уровня магния в крови.
- Снижение уровня кальция в крови.
- Головокружение.
- Головная боль.
- Онемение.
- Диарея.
- Тошнота, рвота.
- Чувство слабости или усталости.
- Высокая температура или озноб.
- Кожная сыпь.
- Изменения в функционировании почек (показано в анализах крови), например, увеличение креатинина, снижение концентрации гемоглобина.
Часто: могут возникать у до 1 из 10 человек
- Низкий уровень белых кровяных клеток (лейкопения и/или нейтропения). Симптомы включают инфекции и высокую температуру (лихорадку).
- Низкий уровень тромбоцитов в крови. Это может сделать более легким появление синяков.
- Общая инфекция (сепсис).
- Увеличение или снижение уровня фосфата в крови.
- Снижение уровня натрия в крови.
- Увеличение уровня кальция в крови.
- Дегидратация.
- Нарушение функционирования печени.
- Увеличение ферментов печени (гамма-ГТ, аланинаминотрансфераза (АЛТ), аспартатаминотрансфераза (АСТ), ЛДГ).
- Изменения настроения. Они включают агрессию, возбуждение, тревогу, путаницу, депрессию, нервозность.
- Нарушение координации движений.
- Судороги.
- Снижение чувствительности кожи.
- Нерегулярные сердечные сокращения (пальпитация).
- Быстрый сердечный ритм (тахикардия).
- Нарушения нервной системы, которые могут вызывать изменения чувствительности или мышечную слабость (нейропатия).
- Дрожь.
- Гипертония.
- Гипотония, которая может вызывать головокружение.
- Отек, боль и покраснение вдоль вены.
- Боль в животе, запор и диспепсия.
- Воспаление поджелудочной железы (панкреатит) или изменения в функционировании поджелудочной железы. Симптомы включают сильную боль в животе и могут быть изменения в анализах крови (увеличение липаз).
- Зуд кожи.
- Боль в мышцах.
- Нарушения функционирования почек, например, боль в почках (боль в нижней части спины), почечная недостаточность, присутствие белка в моче.
- Боль при мочеиспускании.
- Язвы или эрозии гениталий. Это одиночные или множественные повреждения, которые исчезают после прекращения лечения (см. «Предостережения и меры предосторожности»).
- Общее недомогание.
- Боль и воспаление в месте инъекции.
- Отек ног и рук.
- Изменения в результатах тестов, показывающих функционирование сердца (ЭКГ).
Редко: могут возникать у до 1 из 100 человек
- Снижение количества красных, белых кровяных клеток и тромбоцитов.
- Чрезмерная кислотность крови. Это может сделать более быстрым ваше дыхание.
- Зудящая сыпь (уртикария)
- Нарушения функционирования почек и мочевыделительной системы (тубулярное повреждение почек, гломерулонефрит, нефротический синдром).
- Увеличение некоторых ферментов крови, например, амилазы, креатинкиназы и фосфокиназы.
Частота не известна (не может быть оценена по доступным данным)
- Нерегулярные сердечные сокращения.
- Сахарный диабет. Симптомы включают более частое мочеиспускание. Реже, также может быть чрезмерная жажда.
- Увеличение уровня натрия в крови.
- Энцефалопатия.
- Нерегулярные сердечные сокращения или изменения сердечного ритма, например, торсад де поинт.
- Мышечные проблемы: мышечная слабость, разрушение мышечной ткани (рабдомиолиз: аномальный цвет мочи и сильная мышечная жесткость, чувствительность или слабость).
- Проблемы с почками, например, боль, кровь в моче, некроз канальцев, нефропатия, вызванная кристаллами.
- Экстравазация.
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, проконсультируйтесь с вашим врачом или фармацевтом, даже если это возможные побочные эффекты, которые не указаны в этом проспекте. Вы также можете сообщить об этом напрямую через систему фармакологического надзора за лекарствами для человека: https://www.notificaRAM.es. Сообщая о побочных эффектах, вы можете внести свой вклад в предоставление более полной информации о безопасности этого лекарства.
5. Хранение Фоскарнет Кabi
- Храните это лекарство в недоступном для детей месте.
- Не используйте это лекарство после даты истечения срока годности, указанной на этикетке после CAD. Дата истечения срока годности - последний день месяца, указанного.
- Не храните в холодильнике или не замораживайте. Если лекарство хранится в холодильнике или подвергается воздействию температуры ниже точки замерзания, оно может выпасть в осадок. Этот осадок может снова раствориться, если держать флакон при комнатной температуре и встряхивать его несколько раз.
- Химическая и физическая стабильность продукта при использовании была подтверждена в течение 9 дней при 25 °C. С микробиологической точки зрения, продукт должен быть использован сразу же, если только открытие и разбавление не были проведены в условиях, исключающих риск микробной контаминации. Если продукт не используется сразу же, время и условия хранения до использования являются ответственностью пользователя.
- Фоскарнет может быть смешан с другим раствором фармацевтом. Это делается для предоставления вам готового к использованию лекарства. Фармацевт сообщит вам, как хранить и когда использовать лекарство.
- Лекарства не должны быть выбрасываны в канализацию или мусор. Спросите вашего фармацевта, как утилизировать упаковку и лекарства, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
Состав Фоскарнета Кabi
Активное вещество - фоскарнет.
Каждый мл содержит 24 мг фоскарнета содий гидрата.
Остальные компоненты - вода для инъекционных препаратов и соляная кислота.
Внешний вид продукта и содержание упаковки
Фоскарнет - стерильная раствор для инфузии.
Раствор прозрачный и бесцветный.
Фоскарнет выпускается в флаконах по 250 мл.
Владелец разрешения на маркетинг
Fresenius Kabi España, S.A.U
Торре Мапфре – Вила Олимпика
Марина 16-18
08005 Барселона
Испания
Производитель
Fresenius Kabi Austria GmbH
Хафнерштрассе 36
A-8055 Грац
Австрия
Это лекарство разрешено в государствах-членах Европейского экономического пространства под следующими названиями:
Бельгия: Фоскарнет Фрезенius Кabi 24 мг/мл раствор для инфузии; Фоскарнет Фрезенius Кabi 24 мг/мл раствор для перфузии; Фоскарнет Фрезенius Кabi 24 мг/мл инфузионный раствор
Франция: ФОСКАРНЕТ КАБИ 24 мг/мл, раствор для перфузии
Германия: Фоскарнет Кabi 24 мг/мл инфузионный раствор
Италия: Фоскарнет Кabi
Люксембург: Фоскарнет содий 24 мг/мл раствор для инфузии
Португалия: Фоскарнет содий Кabi
Испания: Фоскарнет Кabi 24 мг/мл раствор для перфузии ЕФГ
Великобритания: Фоскарнет содий 24 мг/мл раствор для инфузии
Дата последнего пересмотра этого листка инструкции:август 2022
Подробная информация о этом лекарстве доступна на сайте Агентства по лекарственным средствам и медицинским изделиям Испании (АЕМПС) http://www.aemps.gob.es/.
------------------------------------------------------------------------------------------------------------------------
Эта информация предназначена только для медицинских специалистов:
Обращаться с Фоскарнетом Кabi с осторожностью
- У пациентов с нарушением функции почек:
- Креатинин сыворотки крови должен контролироваться регулярно.
- Необходимо предусмотреть коррекцию дозы у пациентов с нарушенной функцией почек. Для предотвращения изменений функции почек, которые могут возникнуть во время лечения, должна поддерживаться адекватная гидратация.
- Не использовать растворы, которые могут содержать кальций.
- В случае случайного контакта, промыть водой кожу или слизистые оболочки.
Способ введения
Фоскарнет должен вводиться только внутривенно, либо через центральный венозный катетер, либо в периферическую вену.
Предостережение: Не вводить фоскарнет быстро внутривенно.
Не вводить фоскарнет без режима гидратации.
Не использовать растворы, которые могут содержать кальций, глюкозу 30%, амфотерицин Б, ацикловир содий, ганцикловир, изетионат пентамидины, триметоприм-сульфаметоксазол и хлорид ванкомицина.
Как и при всех перфузиях, требуется соблюдение строго асептических условий при обращении.
При использовании периферических вен раствор фоскарнета 24 мг/мл должен быть разбавлен. Службы фармацевтического обеспечения больниц должны асептически перенести индивидуальные дозы фоскарнета в пластиковые мешки для перфузии, а также разбавить его в равных частях хлорида натрия 0,9% (9 мг/мл) или декстрозы 5% (50 мг/мл). Физико-химическая стабильность фоскарнета, его разбавлений и разбавлений в мешках из ПВХ составляет 9 дней. Разбавленные растворы должны использоваться как можно скорее после приготовления, но могут храниться до 24 часов, если они хранятся в холодильнике.
Почечная токсичность фоскарнета может быть снижена при адекватной гидратации пациента. См. раздел «Гидратация» ниже.
Если используется система перфузии Y, необходимо одновременно перфузировать 0,5-1 литр NaCl 0,9% или глюкозы 5%.
Не вводить другие препараты в той же перфузии. Когда продукт вводится перфузией в периферическую вену, одновременная внутривенная гидратация служит разбавлением (см. раздел «Гидратация»).
Схема системы перфузии Y
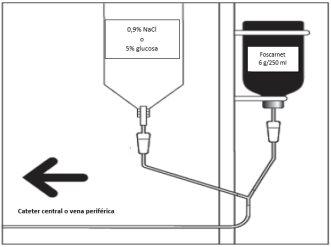
Гидратация:
Необходимо обратить особое внимание на важность предотвращения почечной токсичности фоскарнета, обеспечивая пациентам адекватную гидратацию.
Если используется система перфузии Y, необходимо одновременно перфузировать 0,5-1 литр NaCl 0,9% или глюкозы 5%. У пациентов, соответствующих требованиям, использовалась пероральная гидратация с аналогичными режимами гидратации. Клинически обезвоженные пациенты должны быть корректированы до начала лечения фоскарнетом.
Каждый флакон фоскарнета должен использоваться только для лечения одного пациента с одной перфузией.
Случайный контакт с кожей или глазами раствора фоскарнета содия может вызвать местное раздражение и ощущение жжения. В случае случайного контакта необходимо промыть пораженную область водой.
Фоскарнет, охлажденный или подвергнутый воздействию температур ниже точки замерзания, может выпасть в осадок. При поддержании флакона при комнатной температуре с постоянным встряхиванием осадок может снова раствориться.
Утилизация неиспользованного лекарства и всех материалов, которые были в контакте с ним, должна осуществляться в соответствии с местными правилами.
- Страна регистрации
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги ФОСКАРНЕТ КАБИ 24 мг/мл РАСТВОР ДЛЯ ИНФУЗИЙФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР ДЛЯ ИНФУЗИЙ, 24 мг/млАктивное вещество: foscarnetПроизводитель: Laboratorios Tillomed Spain S.L.UТребуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР ДЛЯ ИНФУЗИЙ, 24 мг фоскарнета натрия/млАктивное вещество: foscarnetПроизводитель: Clinigen Healthcare B.V.Требуется рецептФорма выпуска: ТАБЛЕТКА, 300 мгАктивное вещество: АбакавирПроизводитель: Tarbis Farma S.L.Требуется рецепт
Аналоги ФОСКАРНЕТ КАБИ 24 мг/мл РАСТВОР ДЛЯ ИНФУЗИЙ в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог ФОСКАРНЕТ КАБИ 24 мг/мл РАСТВОР ДЛЯ ИНФУЗИЙ в Польша
Врачи онлайн по ФОСКАРНЕТ КАБИ 24 мг/мл РАСТВОР ДЛЯ ИНФУЗИЙ
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на ФОСКАРНЕТ КАБИ 24 мг/мл РАСТВОР ДЛЯ ИНФУЗИЙ – по решению врача и с учетом местных правил.














