FIBCLOT 1.5 g POWDER AND SOLVENT FOR INJECTABLE SOLUTION AND FOR INFUSION


How to use FIBCLOT 1.5 g POWDER AND SOLVENT FOR INJECTABLE SOLUTION AND FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
FibCLOT 1.5g
Powder and solvent for solution for injection and infusion
human fibrinogen
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is FibCLOT and what is it used for
- What you need to know before you use FibCLOT
- How to use FibCLOT
- Possible side effects
- Storage of FibCLOT
- Contents of the pack and other information
1. What is FibCLOT and what is it used for
What is FibCLOT
It is a medicine that belongs to the group of antihemorrhagics. The active substance is human fibrinogen, a protein naturally present in the body. The function of this protein is to ensure that blood clots normally and prevent bleeding from lasting too long.
What is FibCLOT used for
It is used in all age groups to compensate for the lack of human fibrinogen and thus treat bleeding (hemorrhages) in patients with congenital fibrinogen deficiency.
Congenital fibrinogen deficiency is a hereditary disease characterized by a lower than normal amount or absence of a protein called fibrinogen. This deficiency can cause prolonged bleeding.
2. What you need to know before you use FibCLOT
Do not use FibCLOT
If you are allergic to the active substance (human fibrinogen) or to any of the other components of this medicine (listed in section 6. "Contents of the pack and other information").
Tell your doctor if you are allergic to any medicine.
Warnings and precautions:
Consult your doctor, pharmacist, or nurse before starting treatment with FibCLOT.
Traceability
In order to improve the traceability of biological medicinal products, the name and batch number of the administered product must be clearly recorded.
Risk of blood clots
With high doses or repeated administrations, this medicine may increase the risk of blood clots in the blood vessels.
Therefore, your doctor should weigh the benefits of this medicine against the risk of blood clots, especially:
- If you have had a heart attack (history of coronary heart disease or myocardial infarction).
- If you have liver disease.
- If you have recently undergone surgery.
- If you are going to undergo surgery soon.
- In newborns (neonates).
- If you are more likely than normal to suffer from blood clots.
Your doctor may request additional tests to monitor this risk.
Risk of allergies
Your doctor will inform you of the warning signs of an allergic reaction or a severe allergic reaction (anaphylactic reaction) (see section 4. "Possible side effects"). If any of these effects occur, administration of this medicine should be stopped immediately.
Viral safety
This medicine is made from human plasma (the liquid part of the blood).
When manufacturing medicines from human blood or plasma, certain measures are taken to prevent the transmission of infections to patients. Some of these measures are:
- careful selection of blood and plasma donors to ensure that those at risk of being carriers of infections are excluded,
- testing of each donation and plasma pools for signs of viral infections,
- inclusion of processing steps that can inactivate or remove viruses.
Despite these measures, when administering medicines derived from human blood or plasma, the possibility of transmitting an infection cannot be completely ruled out. This also applies to unknown or emerging viruses and other types of infections.
The measures taken are considered effective against enveloped viruses, such as the human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus, and against the non-enveloped hepatitis A virus.
The measures taken may have limited value against non-enveloped viruses, such as parvovirus B19. Parvovirus B19 infection can be severe in pregnant women (fetal infection) and in individuals with a weakened immune system or with certain types of anemia (e.g., sickle cell disease or hemolytic anemia).
If you receive human plasma-derived products periodically/repeatedly, your doctor may recommend that you consider getting vaccinated against hepatitis A and B.
Immune risk
In the context of replacement therapy with coagulation factors for other congenital deficiencies, immune reactions have been observed, but no data are available for fibrinogen.
Children and adolescents
The warnings and precautions mentioned above are valid for children and adolescents.
Using FibCLOT with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicine, including those obtained without a prescription.
No interactions have been observed between this treatment and other medicines to date. However, it should not be mixed with other products and/or medicines.
Pregnancy and breastfeeding
- If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. This medicine should only be used during pregnancy and breastfeeding if recommended by your doctor.
- Consult your doctor if you discover you are pregnant during treatment, as only your doctor can determine if you need to continue with it.
Driving and using machines
The influence of this medicine on the ability to drive and use machines is zero.
FibCLOT contains sodium.
This medicine contains up to 3 mmol (or 69 mg) of sodium (the main component of kitchen/table salt) per vial. This amount is equivalent to 3.45% of the maximum daily recommended intake of sodium for an adult.
If you are on a low-salt diet, you should be aware of this.
3. How to use FibCLOT
Treatment should be initiated under the supervision of a doctor experienced in the treatment of congenital fibrinogen deficiency.
Dose
Your doctor will determine the appropriate dose and frequency, which will depend on the following factors:
- your body weight,
- the severity of your disorder,
- the location and extent of bleeding, or the nature of your surgical procedure,
- your state of health.
Your doctor will recommend that you have blood tests during treatment to check the amount of fibrinogen in your body.
Depending on the test results, your doctor may decide to adjust the dose and frequency of injections.
Frequency of administration
Your doctor will determine how often the injections should be administered.
In addition, your doctor will adjust the number of injections based on the intensity of bleeding and the effectiveness of treatment.
In the section reserved for healthcare professionals at the end of this leaflet, information is included on the frequency and duration of treatment in various situations.
Method of administration:
This medicine must be injected into a vein. The use of an infusion set with a 15 µm filter, such as the one supplied with the pack, is mandatory.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
If you use more FibCLOT than you should
To avoid the risk of overdose, your doctor will perform periodic blood tests to check the amount of fibrinogen in your body.
In case of overdose, the risk of abnormal blood clot formation cannot be ruled out.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The most serious side effects are:
- Allergic reactions:as with any intravenous protein product, allergic reactions can occur. In some cases, these reactions have worsened to become a severe allergic reaction, sometimes with a marked drop in blood pressure (anaphylactic shock).
The warning signs of allergic reactions are:
- burning sensation and tingling at the injection site,
- tingling,
- redness, itching, and rash,
- hives,
- skin inflammation,
- pallor,
- swelling of the face or throat,
- cough,
- wheezing (similar to asthma),
- chest tightness,
- rapid heartbeat,
- low blood pressure,
- extreme fatigue (lethargy),
- restlessness,
- chills,
- feeling of illness (nausea), vomiting,
If you experience any of these effects, contact a doctor immediately, who will stop treatment with this medicine and/orinitiate appropriate treatment depending on the type and severity of the reaction. |
- Blood clots: blood clots can form in the bloodstream. These can cause:
- a heart attack, whose warning signs are sudden chest pain or difficulty breathing;
- a stroke, whose warning signs are sudden muscle weakness, loss of sensation and/or balance, decreased alertness, or difficulty speaking;
- a serious disease called pulmonary embolism (blood clot that blocks an artery in the lungs), whose warning signs are chest pain, difficulty breathing, or coughing up blood;
- a clot in a vein (venous thrombosis), whose warning signs are redness, warmth, pain, pain on palpation, or swelling in one or both legs.
If you experience any of these effects, contact a doctor immediately, who will stop treatment with this medicine and/orinitiate appropriate treatment depending on the type and severity of the reaction. |
The following side effects are common(may affect up to 1 in 10 infusions):
- headache.
The following side effects are uncommon(may affect up to 1 in 100 infusions):
- allergic reaction (such as anaphylactic shock, pallor, vomiting, cough, low blood pressure, chills, urticarial rash [hives]; see also the section "Allergic reactions"),
- dizziness,
- vomiting (associated with headache)
- ringing in the ears,
- blood circulation disorders (deep vein thrombosis, superficial phlebitis),
- breathing difficulties (asthma),
- skin rash, redness of the skin, skin irritation, night sweats,
- feeling of heat.
Children and adolescents
The frequency, type, and severity of side effects are similar in pediatric patients (from birth to 17 years old) and adults, except for allergic reactions or anaphylactic reactions, which occurred more frequently in the pediatric population.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of FibCLOT
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton. The expiry date is the last day of the month stated.
Do not store above 25°C. Do not freeze.
Keep the vial in the outer packaging to protect it from light and moisture.
The product should be used immediately after reconstitution. Do not store the reconstituted product.
Do not use this medicine if the reconstituted solution is cloudy or contains sediment.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
FibCLOT Composition
The active ingredient is human fibrinogen (1.5 g per vial). After reconstitution with 100 mL of water for injectable preparations, FibCLOT contains 15 mg/mL of human fibrinogen.
The other components are arginine hydrochloride, isoleucine, lysine hydrochloride, glycine, sodium citrate dihydrate, and solvent (water for injectable preparations).
Product Appearance and Container Contents
This medication is presented in powder form accompanied by a solvent for solution for injectable preparations in glass vials, a transfer device, and an infusion device with a 15 µm filter.
The reconstituted solution should be practically colorless, slightly opalescent (it should have a pearlescent sheen).
Marketing Authorization Holder:
Laboratoire français du Fractionnement et des Biotechnologies
Tour W – 102 Boieldieu 19ème Étage, 92800 Puteaux, FRANCE
Tel.: +33(0) 1 69 82 70 10
Fax: +33(0) 1 69 82 19 03
Manufacturer:
LFB BIOMEDICAMENTS
59 rue de Trévise, 59000 Lille, FRANCE
For further information about this medicinal product, please contact the local representative of the marketing authorization holder:
LFB BIOTERAPIAS HISPANIA
C/ Diego de León 47
28006 Madrid
(Spain)
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Germany, Austria, Spain, Greece, United Kingdom: FibCLOT
Denmark, Finland, Hungary, Luxembourg, Norway, Netherlands, Sweden: Fibclot
Belgium: Fibclot 1.5 g, powder and solvent for solution for injection/infusion
Italy: Fibriclotte
Date of last revision of this leaflet: 06/2024
---------------------------------------------------------------------------------------------------------------------------
This information is intended for healthcare professionals only:
Treatment should be initiated under the supervision of a physician experienced in the treatment of coagulation disorders.
Dosage
The dose and duration of substitute therapy depend on the severity of the disorder, the location and magnitude of the hemorrhage, as well as the patient's clinical condition.
The fibrinogen concentration (functional) should be determined in order to calculate the individual dose; in addition, the amount and frequency of administration should be calculated for each specific patient through periodic determination of plasma fibrinogen concentration and continuous monitoring of the patient's clinical condition and other substitute therapies used.
The normal concentration of fibrinogen in plasma ranges from 1.5 to 4.5 g/L. In congenital hypo or afibrinogenemia, the critical fibrinogen concentration in plasma below which bleeding may occur is approximately between 0.5 and 1.0 g/L.
In the case of major surgical intervention, it is essential to monitor substitute therapy through coagulation tests.
Treatment of hemorrhages and perioperative prophylaxis in patients with congenital hypo or afibrinogenemia and known bleeding tendency.
For the treatment of non-surgical hemorrhagic episodes, it is recommended to increase the fibrinogen concentration to 1 g/L and maintain this concentration until hemostasis is controlled, and above 0.5 g/L until healing is complete.
To avoid excessive bleeding during surgical procedures, prophylactic treatment is recommended to increase the fibrinogen concentration to 1 g/L and maintain this concentration until hemostasis is controlled, and above 0.5 g/L until healing is complete.
In the case of a surgical procedure or the treatment of a non-surgical hemorrhage, the dose should be calculated as follows:
Dose (g) = [desired concentration (g/L) - baseline concentration (g/L)] x 1/recovery (g/L) (g/kg) x body weight (kg),
The "1/recovery" ratio is defined by the patient's recovery* (see section 5.2 of the datasheet), or if recovery is unknown:
- 0.053 (g/kg)/(g/L) for children and adolescents <40 kg body weight< li>
- 0.043 (g/kg)/(g/L) for adults and adolescents ≥40 kg body weight.
- Example for patient recovery and dose calculation
For a 60 kg patient with a baseline fibrinogen concentration of zero and an increase in fibrinogen to 1.20 g/L 1 hour after infusion of 0.060 g/kg of FibCLOT:
- Calculation of patient recovery:
1.20 (g/L) / 0.060 (g/kg) = 20.0 (g/L)/(g/kg)
- Calculation of dose for an increase to 1.0 g/L:
1.0 g/L x 1 / 20.0 (g/L)/(g/kg) [or 0.050 (g/kg)/(g/L)]x 60 kg = 3 g
In the event of an emergency situation where the baseline fibrinogen concentration is unknown, the recommended initial dose is 0.05 g/kg body weight administered intravenously in adults and adolescents ≥40 kg body weight, and 0.06 g/kg body weight in pediatric patients <40 kg body weight.< p>
Subsequent dosing (dose and frequency of injections) should be adapted based on the patient's clinical condition and analytical results.
The biological half-life of fibrinogen is between 3 and 4 days. Therefore, if there is no consumption, repeated treatment with human fibrinogen is usually not necessary. Taking into account the accumulation that occurs with repeated administration for prophylactic purposes, the dose and frequency should be determined based on the therapeutic objectives of the physician for each individual patient.
Pediatric Population
Recovery and half-life in children and adolescents <40 kg body weight are lower than those in adults and adolescents ≥40 (see section 5.2 of the datasheet). consequently, adapted recoveries should be used to calculate dose fibclot respective groups when individual patient's recovery is unknown. it can assumed that a <40 covers age range from birth approximately 12 years age. dosing regimen (dose frequency injections) according clinical response.< p>
Reconstitution:
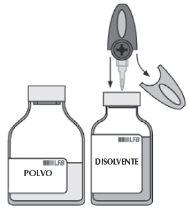
Follow current guidelines for aseptic procedures.
| If necessary, warm the two vials (powder and solvent) to room temperature. |
| Remove the protective cap from the solvent vial and the powder vial. Disinfect the surface of both stoppers. |
| Remove the translucent protective sleeve from the transfer device and, with a rotary motion, fully insert the exposed spike into the center of the solvent vial stopper. |
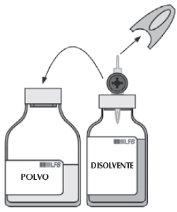
| Remove the second gray protective sleeve from the other end of the transfer device. Rotate the solvent vial and quickly insert the free end of the spike into the center of the powder vial stopper to allow the solvent to reach the powder. Ensure that the spike remains submerged at all times in the solvent to avoid the vacuum disappearing prematurely. |
| During transfer, perform a horizontal rotary motion so that the solvent jet is distributed over the entire surface of the powder and the vial wall. Ensure that all solvent is transferred. The vacuum is automatically eliminated at the end of the transfer procedure by the sterile air that passes through the transfer device's vent. |
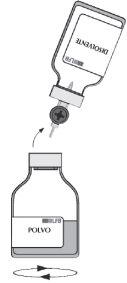
| Remove the empty vial (solvent) with the transfer device. To avoid foaming, gently agitate for a few minutes with a rotary motion until the powder is completely dissolved. |
Before administering the reconstituted product, it should be visually examined to ensure it does not contain particles. The reconstituted solution should be practically colorless and slightly opalescent. Do not use turbid or sediment-containing solutions.
Administration:
FibCLOT should be administered solely by the intravenous route, as a single dose, immediately after reconstitution, and at a rate not exceeding 4 mL/min.
Use of an infusion device with a 15 µm filter, such as the one supplied with the packaging, is mandatory.
Disposal of unused medicinal product and all materials that have come into contact with it should be carried out in accordance with local regulations.
This medicinal product should not be mixed with other medicinal products and should be administered with a separate injection/infusion catheter.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FIBCLOT 1.5 g POWDER AND SOLVENT FOR INJECTABLE SOLUTION AND FOR INFUSIONDosage form: INJECTABLE, 1 gActive substance: fibrinogen, humanManufacturer: Octapharma S.A.Prescription requiredDosage form: INJECTABLE, 1 g (20 mg/mL)Active substance: fibrinogen, humanManufacturer: Csl Behring GmbhPrescription requiredDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription required
Online doctors for FIBCLOT 1.5 g POWDER AND SOLVENT FOR INJECTABLE SOLUTION AND FOR INFUSION
Discuss questions about FIBCLOT 1.5 g POWDER AND SOLVENT FOR INJECTABLE SOLUTION AND FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions