EYLEA 40 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar EYLEA 40 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente adulto
Eylea 40 mg/ml solución inyectable en jeringa precargada
aflibercept
ADULTOS
Si desea información para los tutores de bebés nacidos prematuramente, consulte al final de la sección 6”
Lea todo el prospecto detenidamente antes de que le administren este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico.
- Si experimenta efectos adversos, consulte a su médico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Eylea y para qué se utiliza
- Qué necesita saber antes de que le administren Eylea
- Cómo se le administrará Eylea
- Posibles efectos adversos
- Conservación de Eylea
- Contenido del envase e información adicional
1. Qué es Eylea y para qué se utiliza
Eylea es una solución que se inyecta en el ojo para tratar unas enfermedades oculares en pacientes adultos, denominadas:
- degeneración macular asociada a la edad neovascular (exudativa) comunmente conocida como DMAE exudativa
- alteración de la visión debida al edema macular a causa de un bloqueo de las venas retinianas (oclusión de la vena central de la retina (OVCR) o de la rama venosa de la retina (ORVR))
- alteración de la visión debida al edema macular diabético (EMD)
- alteración de la visión debida a la neovascularización coroidea miópica (NVC miópica).
Aflibercept, el principio activo de Eylea, bloquea la actividad de un grupo de factores denominados factor de crecimiento endotelial vascular A (VEGF-A) y factor de crecimiento placentario (PlGF).
En pacientes con DMAE exudativa y NVC miópica, cuando estos factores existen en cantidad excesiva influyen en la formación anómala de nuevos vasos sanguíneos en el ojo. Estos nuevos vasos sanguíneos pueden causar una fuga de los componentes de la sangre hacia el interior del ojo, con el consiguiente daño en los tejidos oculares responsables de la visión.
En pacientes con OVCR, se produce un bloqueo de la vena principal que transporta sangre desde la retina. A causa de ello, los niveles de VEGF aumentan causando la fuga de fluido en la retina y por tanto, la hinchazón de la mácula (la parte de la retina responsable de la visión fina), lo cual se conoce como edema macular.
Cuando la mácula se llena de líquido, la visión central se vuelve borrosa.
En pacientes con ORVR, se produce un bloqueo de una o más ramas del vaso sanguíneo principal que transporta sangre desde la retina. A causa de ello, los niveles de VEGF aumentan causando la fuga de líquido en la retina y, por tanto, la hinchazón de la mácula.
El edema macular diabético es una hinchazón de la retina que se produce en pacientes con diabetes debido a la fuga de líquido de los vasos sanguíneos de la mácula. La mácula es la parte de la retina responsable de la visión fina. Cuando la mácula se hincha de líquido, la visión central se vuelve borrosa.
Eylea ha demostrado detener el crecimiento de los nuevos vasos sanguíneos anómalos en el ojo que a menudo sangran o presentan fugas de líquido. Eylea puede ayudar a estabilizar y, en muchos casos, a mejorar la pérdida de visión producida por la DMAE exudativa, OVCR, ORVR, EMD y NVC miópica.
2. Qué necesita saber antes de que le administren Eylea
No le deben administrar Eylea
- si es alérgico a aflibercept o a alguno de los demás componentes de este medicamento (incluidos en la sección 6)
- si tiene una infección activa o sospecha que pueda tener una infección en el ojo o a su alrededor (infección ocular o periocular)
- si padece una inflamación grave del ojo (indicada por dolor o enrojecimiento).
Advertencias y precauciones
Consulte a su médico antes de que le administren Eylea:
- Si sufre glaucoma.
- Si tiene antecedentes de visión de destellos de luz o partículas flotantes o si de repente aumenta el tamaño y número de partículas flotantes.
- Si le han operado o tiene programada una cirugía en su ojo en las cuatro semanas previas o en las cuatro semanas siguientes.
- Si padece una forma grave de OVCR o bien ORVR (OVCR u ORVR isquémicas), no está recomendado el tratamiento con Eylea.
Además, es importante que sepa que:
- La seguridad y eficacia de Eylea cuando se administra en ambos ojos a la vez no se ha estudiado y si se utiliza de esta forma puede dar lugar a un mayor riesgo de que se produzcan efectos adversos.
- Las inyecciones de Eylea pueden producir un aumento de la presión dentro del ojo (presión intraocular) en algunos pacientes en los 60 minutos siguientes a la inyección. Su médico le realizará un seguimiento después de cada inyección.
- Si desarrolla una infección o inflamación en la parte interna del ojo (endoftalmitis) u otras complicaciones, puede notar dolor o un aumento de las molestias en el ojo, un empeoramiento del enrojecimiento ocular, visión borrosa o disminuida y aumento de la sensibilidad a la luz. Es importante que todo síntoma que aparezca se diagnostique y se trate lo antes posible.
- Su médico comprobará si tiene otros factores de riesgo que puedan aumentar la posibilidad de que se produzca un desgarro o un desprendimiento de las capas posteriores del ojo (desgarro o desprendimiento de retina, o bien un desgarro o desprendimiento del epitelio pigmentario de la retina) en cuyo caso Eylea se le administrará con precaución.
- Eylea no se debe utilizar durante el embarazo, a menos que el beneficio potencial supere el riesgo potencial para el feto.
- Las mujeres en edad fértil deben utilizar métodos anticonceptivos efectivos durante el tratamiento y durante al menos tres meses más después de la última inyección de Eylea.
El uso sistémico de inhibidores del VEGF, sustancias parecidas a las que contiene Eylea, está potencialmente relacionado con el riesgo de bloqueo de los vasos sanguíneos por coágulos de sangre (acontecimientos tromboembólicos arteriales) que pueden dar lugar a un infarto de miocardio o un accidente cerebrovascular. Tras la inyección de Eylea en el ojo, existe un riesgo teórico de que se puedan producir estos acontecimientos. Los datos sobre la seguridad del tratamiento de pacientes con OVCR, ORVR, EMD y NVC miópica que han sufrido un accidente cerebrovascular, un accidente cerebrovascular transitorio (ataque isquémico transitorio), o bien un infarto de miocardio en los últimos 6 meses son limitados. Si alguno de estos casos le aplica, se le administrará Eylea con precaución.
La experiencia es solo limitada en el tratamiento de:
- Pacientes con EMD debido a diabetes de tipo I.
- Pacientes diabéticos con valores medios de azúcar en sangre muy elevados (Hemoglobina glicosilada superior al 12%).
- Pacientes diabéticos con una enfermedad ocular provocada por la diabetes, conocida como retinopatía diabética proliferativa.
No existe experiencia en el tratamiento de:
- Pacientes con infecciones agudas.
- Pacientes con otras enfermedades oculares como desprendimiento de retina o agujero macular.
- Pacientes diabéticos con hipertensión no controlada.
- Pacientes no asiáticos con NVC miópica.
- Pacientes que han sido tratados anteriormente por una NVC miópica.
- Pacientes con daños fuera de la parte central de la mácula (lesiones extrafoveales) debido a una NVC miópica.
Si algo de lo anterior le sucede, su médico tendrá en cuenta esta falta de información en el momento de tratarle con Eylea.
Niños y adolescentes
No se ha estudiado el uso de Eylea en niños y adolescentes menores de 18 años para indicaciones distintas de retinopatía del prematuro (ROP).
Otros medicamentos y Eylea
Informe a su médico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
- Las mujeres en edad fértil deben utilizar métodos anticonceptivos efectivos durante el tratamiento y durante al menos tres meses más después de la última inyección de Eylea.
- No hay experiencia con el uso de Eylea en mujeres embarazadas. No se debe utilizar Eylea durante el embarazo a menos que el beneficio potencial supere al riesgo potencial para el feto. Si está embarazada o tiene intención de quedarse embarazada, coméntelo con su médico antes del tratamiento con Eylea.
- Pueden pasar a la leche materna cantidades pequeñas de Eylea. Se desconocen los efectos en recién nacidos/bebés lactantes. Eylea no está recomendado durante la lactancia. Si usted es una mujer en periodo de lactancia, coméntelo con su médico antes del tratamiento con Eylea.
Conducción y uso de máquinas
Después de la inyección de Eylea puede experimentar algunas alteraciones visuales transitorias. No conduzca ni use máquinas mientras duren estas alteraciones.
Eylea contiene
- menos de 1 mmol de sodio (23 mg) por unidad de dosis; esto es, esencialmente “exento de sodio”.
- 0,015 mg de polisorbato 20 en cada dosis de 0,05 ml equivalente a 0,3 mg/ml. Los polisorbatos pueden causar reacciones alérgicas. Informe a su médico si tiene cualquier alergia conocida.
3. Cómo se le administrará Eylea
Eylea le será administrado por un médico con experiencia en la administración de inyecciones oculares, en condiciones asépticas (de limpieza y estériles).
La dosis recomendada es de 2 mg de aflibercept (0,05 ml).
Eylea se administra en forma de inyección en el interior del ojo (inyección intravítrea).
Antes de la inyección, su médico utilizará un lavado ocular desinfectante para limpiar cuidadosamente su ojo para prevenir una infección. Su médico también le administrará un anestésico local para reducir o prevenir cualquier dolor que pudiera sentir con la inyección.
DMAE exudativa
Los pacientes con DMAE exudativa se tratarán con una inyección mensual para las tres primeras dosis, seguido de otra inyección después de otros dos meses.
Su médico decidirá entonces si el intervalo de tratamiento entre las inyecciones puede mantenerse cada dos meses o extenderse gradualmente en intervalos de 2 o 4 semanas si su enfermedad se ha estabilizado. Si su enfermedad empeora, el intervalo entre las inyecciones puede acortarse.
No es necesario que su médico le visite entre inyecciones, a menos que su médico considere lo contrario o usted experimente algún problema.
Edema macular secundario a OVR (de rama o central)
Su médico determinará el programa de tratamiento más adecuado para usted. Su tratamiento se iniciará con una serie de inyecciones de Eylea administradas una vez al mes.
El intervalo entre dos inyecciones no debe ser inferior a un mes.
Su médico podrá decidir interrumpir el tratamiento con Eylea si no se beneficia del tratamiento continuado.
El tratamiento continuará con una inyección una vez al mes hasta que su enfermedad se estabilice. Puede necesitar tres o más inyecciones mensuales.
Su médico controlará su respuesta al tratamiento y podrá continuar el tratamiento, incrementando de forma gradual el intervalo entre las inyecciones para estabilizar su enfermedad. En caso de empeoramiento con un intervalo entre tratamientos más largo, su médico reducirá el intervalo entre inyecciones.
En función de su respuesta al tratamiento, su médico decidirá el programa de seguimiento y tratamiento.
Edema macular diabético (EMD)
Los pacientes con EMD se tratarán con una inyección mensual para las cinco primeras dosis consecutivas, y a continuación, una inyección cada dos meses.
El intervalo entre tratamientos puede mantenerse cada dos meses o ajustarse según su enfermedad en función de la exploración realizada por su médico. Su médico decidirá el programa de visitas de seguimiento.
Su médico podrá decidir la interrupción del tratamiento con Eylea si comprueba que usted no se beneficia del tratamiento continuado.
Neovascularización coroidea (NVC) miópica
Los pacientes con NVC miópica serán tratados con una sola inyección. Solamente recibirá más inyecciones si las exploraciones de su médico revelan que su enfermedad no ha mejorado.
El intervalo entre dos inyecciones no debe ser inferior a un mes.
Si su enfermedad desaparece y luego regresa, su médico puede reiniciar el tratamiento.
Su médico decidirá sobre el programa de revisiones de seguimiento.
Se presentan instrucciones detalladas para el uso al final de este prospecto en “Cómo preparar y administrar Eylea a adultos”.
Si no se le administra una dosis de Eylea
Pida una nueva cita para que le examinen y le administren la inyección.
Interrupción del tratamiento con Eylea
Consulte a su médico antes de interrumpir el tratamiento.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Potencialmente podrían producirse reacciones alérgicas(hipersensibilidad). Estas pueden ser graves yrequerir que se ponga en contacto con su médico inmediatamente.
Con la administración de Eylea pueden producirse algunos efectos adversos que afectan a los ojos que son debidos al procedimiento de inyección. Algunos pueden ser graves, incluyendo ceguera, unainfeccióngrave o inflamación en el interior del ojo(endoftalmitis), desprendimiento, desgarro o hemorragia de la capa sensible a la luz en la parte posterior del ojo(desprendimiento o desgarro de la retina), enturbiamiento del cristalino(catarata), hemorragia en el ojo(hemorragia vítrea), desprendimiento de la sustancia similar a un gel que se encuentra en el interior del ojo en contacto con la retina(desprendimiento de vítreo) y aumento de la presión en el interior del ojo(ver sección 2). Estos efectos adversos graves que afectan a los ojos se produjeron en menos de 1 de 1.900 inyecciones durante los ensayos clínicos.
Si nota una disminución repentina de la visión o un aumento del dolor y enrojecimiento en el ojo después de la inyección, consulte inmediatamente a su médico.
Lista de los efectos adversos comunicados
A continuación se incluye una lista de los efectos adversos comunicados como posiblemente relacionados con el procedimiento de inyección o con el medicamento. No debe alarmarse, ya que puede que usted no experimente ninguno de ellos. Consulte siempre con su médico acerca de cualquier sospecha de efecto adverso.
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- deterioro de la visión
- sangrado en la parte posterior del ojo (hemorragia retiniana)
- sangre en el ojo debido al sangrado de pequeños vasos sanguíneos en las capas externas del ojo
- dolor ocular
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas):
- desprendimiento o desgarro de una de las capas de la parte posterior del ojo que producen destellos de luz con manchas flotantes que en ocasiones progresa a pérdida de visión (desgarro*/desprendimiento del epitelio pigmentario de la retina, desgarro/desprendimiento de la retina)
- *Efectos adversos que se sabe están asociados a la DMAE exudativa; observados únicamente en pacientes con DMAE exudativa.
- degeneración de la retina (que causa alteraciones de la visión)
- sangrado en el ojo (hemorragia vítrea)
- ciertas formas de enturbiamiento del cristalino (catarata)
- daños en la capa superficial del globo ocular (la córnea)
- aumento de la presión en el interior del ojo
- manchas en la visión (partículas flotantes)
- desprendimiento de la sustancia similar a un gel que se encuentra en el interior del ojo de la retina (desprendimiento vítreo, que resulta en destellos de luz con manchas flotantes)
- sensación de tener algo dentro del ojo
- aumento de la producción de lágrimas
- hinchazón del párpado
- sangrado en el lugar de inyección
- enrojecimiento del ojo
Efectos adversos poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- reacciones alérgicas (hipersensibilidad)**
- **Se notificaron reacciones alérgicas como erupción, picor (prurito), ronchas (urticaria) y algunos casos de reacciones alérgicas (anafilácticas/anafilactoides) graves.
- inflamación o infección grave dentro del ojo (endoftalmitis)
- inflamación del iris o de otras partes del ojo (iritis, uveítis, iridociclitis, células flotantes en la cámara anterior)
- sensación anormal en el ojo
- irritación en el párpado
- hinchazón de la capa superficial del globo ocular (córnea)
Efectos adversos raros(pueden afectar hasta 1 de cada 1.000 personas)
- ceguera
- enturbiamiento del cristalino debido a lesión (catarata traumática)
- inflamación de la sustancia similar a un gel que se encuentra en el interior del ojo pus en el ojo
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- inflamación de la parte blanca del ojo asociada con enrojecimiento y dolor (escleritis)
En los ensayos clínicos se observó un aumento de la incidencia de sangrado de los vasos sanguíneos pequeños en las capas externas del ojo (hemorragia conjuntival) en pacientes con DMAE exudativa que recibían tratamiento con medicamentos anticoagulantes. Este aumento de la incidencia fue comparable en los pacientes tratados con ranibizumab y con Eylea.
El uso de inhibidores del VEGF por vía sistémica, sustancias similares a las contenidas en Eylea, está potencialmente relacionado con el riesgo de formación de coágulos de sangre que bloquean los vasos sanguíneos (eventos tromboembólicos arteriales) que pueden producir un ataque al corazón o una embolia. Hay un riesgo teórico de que pueda producirse este tipo de eventos después de la inyección de Eylea en el ojo.
Al igual que con todas las proteínas terapéuticas, existe la posibilidad de una reacción inmune (formación de anticuerpos) con Eylea.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Eylea
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en la etiqueta después de “CAD/EXP”. La fecha de caducidad es el último día del mes que se indica.
- Conservar en nevera (entre 2 ºC y 8 ºC). No congelar.
- El blíster sin abrir puede conservarse fuera de la nevera por debajo de 25 °C durante un máximo de 24 horas.
- Conservar en el embalaje original para protegerlo de la luz.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Eylea
- El principio activo es: aflibercept. Una jeringa precargada contiene un volumen extraíble de al menos 0,09 ml, equivalente a al menos 3,6 mg de aflibercept. Una jeringa precargada proporciona una dosis de 2 mg de aflibercept en 0,05 ml.
- Los demás componentes son: polisorbato 20 (E 432), dihidrógenofosfato de sodio monohidrato (para el ajuste del pH), hidrogenofosfato de disodio heptahidrato (para el ajuste del pH), cloruro de sodio, sacarosa, agua para preparaciones inyectables.
Ver “Eylea contiene” en la sección 2 para más información.
Aspecto del producto y contenido del envase
Eylea es una solución inyectable (inyectable) en una jeringa precargada. La solución es de incolora a amarillo pálido.
Envase con 1 jeringa precargada.
Titular de la autorización de comercialización
Bayer AG
51368 Leverkusen
Alemania
Responsable de la fabricación
Bayer AG
Müllerstraße 178
13353 Berlin
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België / Belgique / Belgien Bayer SA-NV Tél/Tel: +32-(0)2-535 63 11 | Lietuva UAB Bayer Tel: +370-5-233 68 68 |
| Luxembourg / Luxemburg Bayer SA-NV Tél/Tel: +32-(0)2-535 63 11 |
Ceská republika Bayer s.r.o. Tel: +420-266 101 111 | Magyarország Bayer Hungária KFT Tel: +36-1-487 4100 |
Danmark Bayer A/S Tlf: +45-45 235 000 | Malta Alfred Gera and Sons Ltd. Tel: +356-21 44 62 05 |
Deutschland Bayer Vital GmbH Tel: +49-(0)214-30 513 48 | Nederland Bayer B.V. Tel: +31–23-799 1000 |
Eesti Bayer OÜ Tel: +372-655 85 65 | Norge Bayer AS Tlf: +47-23 13 05 00 |
Ελλ?δα Bayer Ελλ?ς ΑΒΕΕ Τηλ: +30-210-618 75 00 | Österreich Bayer Austria Ges. m. b. H. Tel: +43-(0)1-711 460 |
España Bayer Hispania S.L. Tel: +34-93-495 65 00 | Polska Bayer Sp. z o.o. Tel: +48-22-572 35 00 |
France Bayer HealthCare Tél (N° vert): +33-(0)800 87 54 54 | Portugal Bayer Portugal, Lda. Tel: +351-21-416 42 00 |
Hrvatska Bayer d.o.o. Tel: + 385-(0)1-6599 900 | România SC Bayer SRL Tel: +40-(0)21-529 59 00 |
Ireland Bayer Limited Tel: +353-(0)1-216 3300 | Slovenija Bayer d. o. o. Tel: +386-(0)1-58 14 400 |
Ísland Icepharma hf. Sími: +354-540 80 00 | Slovenská republika Bayer, spol. s r.o. Tel: +421-(0)2-59 21 31 11 |
Italia Bayer S.p.A. Tel: +39-02-3978 1 | Suomi/Finland Bayer Oy Puh/Tel: +358-(0)20-78521 |
Κ?προς NOVAGEM Limited Τηλ: +357-22-48 38 58 | Sverige Bayer AB Tel: +46-(0)8-580 223 00 |
Latvija SIA Bayer Tel: +371-67 84 55 63 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu.
Si desea información local, escanee aquí para acceder al sitio web https://www.pi.bayer.com/eylea1.
Se incluye un código QR con el enlace al prospecto.
<------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
Cómo preparar y administrar Eylea a adultos
La jeringa precargada debe utilizarse para el tratamiento de un solo ojo.
No abrir el blíster con la jeringa precargada estéril fuera de la sala limpia.
La jeringa precargada contiene más cantidad que la dosis recomendada de 2 mg de aflibercept (equivalente a 0,05 ml). El exceso de volumen debe eliminarse antes de la administración.
Antes de la administración, la solución debe inspeccionarse visualmente para detectar la presencia de partículas y/o un cambio de color o cualquier cambio en el aspecto físico. Si observa cualquiera de ellos, no utilice el medicamento.
El blíster sin abrir puede conservarse fuera de la nevera por debajo de 25 °C durante un máximo de 24 horas. Tras la apertura del blíster, proceda bajo condiciones asépticas.
Para la inyección intravítrea debe usarse una aguja de inyección de 30 G x ½ pulgada (1,27 cm).
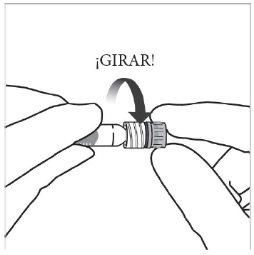
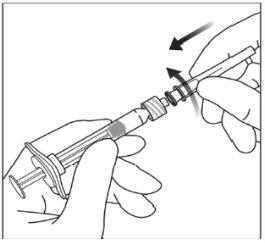
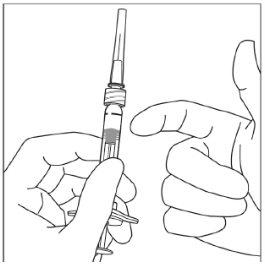
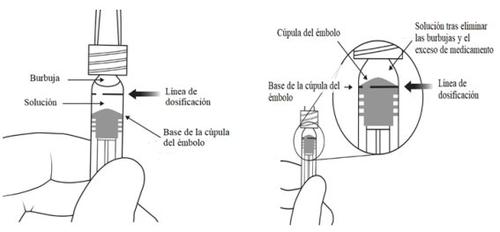
Instrucciones de uso de la jeringa precargada:
| |
| |
|
|
| |
|
|
|
|
Nota:Este posicionamiento exacto del émbolo es muy importante, porque un posicionamiento incorrecto del émbolo puede hacer que se administre más o menos de la dosis recomendada. | |
| |
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local. |
Prospecto: información para los tutores de bebés nacidos prematuramente
Eylea 40mg/ml solución inyectable en jeringa precargada
aflibercept
BEBES NACIDOS PREMATURAMENTE
Lea todo el prospecto detenidamente antes de que le administren este medicamento al bebé, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte al médico del bebé.
- Si observa algún síntoma de efectos adversos, consulte al médico del bebé, incluso si se trata de síntomas y efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Eylea y para qué se utiliza
- Qué necesita saber antes de que le administren Eylea al bebé
- Cómo se le administrará Eylea al bebé
- Posibles efectos adversos
- Conservación de Eylea
- Contenido del envase e información adicional
- Qué es Eylea y para qué se utiliza
Eylea es una solución que se inyecta en el ojo. Eylea pertenece a un grupo de medicamentos denominados agentes antineovascularización. Contiene el principio activo denominado aflibercept.
Eylea se utiliza en bebés nacidos prematuramente para tratar un trastorno ocular denominado retinopatía del prematuro (ROP). Los bebés con ROP tienen un crecimiento anómalo de nuevos vasos sanguíneos en la parte posterior del ojo (retina) inducido por el factor de crecimiento endotelial vascular (VEGF). Esto puede causar afectación de la visión y, en casos graves, ceguera permanente.
Aflibercept, el principio activo de Eylea, bloquea la actividad de un grupo de factores denominados factor de crecimiento endotelial vascular A (VEGF-A) y factor de crecimiento placentario (PlGF).
Eylea ha demostrado detener el crecimiento de los nuevos vasos sanguíneos anómalos en el ojo que a menudo presentan fugas de líquido o sangran. Eylea puede ayudar a estabilizar y, en muchos casos, a mejorar la pérdida de visión producida por la ROP.
- Qué necesita saber antes de que le administren Eylea al bebé
No le deben administrar Eylea al bebé
- si es alérgicoa aflibercept o a alguno de los demás componentes de este medicamento (incluidos en la sección 6)
- si tiene una infección activa o sospecha que pueda tener una infección en el ojo o a su alrededor (infección ocular o periocular)
- si padece una inflamación grave del ojo (indicada por dolor o enrojecimiento).
Advertencias y precauciones
Consulte al médico del bebé antes de que le administren Eylea
- Si han operado al bebé o tiene programada una cirugía en su ojo en las cuatro semanas previas o en las cuatro semanas siguientes.
Además, es importante que sepa que
- Las inyecciones de Eylea pueden producir un aumento de la presión dentro del ojo (presión intraocular) en algunos pacientes en los 60 minutos siguientes a la inyección. El médico del bebé le realizará un seguimiento después de cada inyección.
- Si el bebé desarrolla una infección o inflamación en la parte interna del ojo (endoftalmitis) u otras complicaciones, el bebé puede presentar enrojecimiento/irritación del ojo, secreción ocular, hinchazón del párpadoy aumento de la sensibilidad a la luz. Es importante que todo síntoma que aparezca se diagnostique y se trate lo antes posible.
Informe inmediatamente al médico del bebé si presenta los signos o síntomas descritos.
- El médico del bebé comprobará si tiene otros factores de riesgo que puedan aumentar la posibilidad de que se produzca un desgarro o un desprendimiento de una de las capas posteriores del ojo (desgarro o desprendimiento de retina) en cuyo caso Eylea se le administrará con precaución.
El uso sistémico de inhibidores del VEGF, sustancias parecidas a las que contiene Eylea, está potencialmente relacionado con el riesgo de bloqueo de los vasos sanguíneos por coágulos de sangre (acontecimientos tromboembólicos arteriales) que pueden dar lugar a un infarto de miocardio o un accidente cerebrovascular. Tras la inyección de Eylea en el ojo, existe un riesgo teórico de que se puedan producir estos acontecimientos.
No existe experiencia en el tratamiento de:
- Pacientes con infecciones agudas.
- Pacientes con otras enfermedades oculares como desprendimiento de retina o agujero macular.
Si algo de lo anterior le sucede al bebé, el médico del bebé tendrá en cuenta esta falta de información en el momento de tratarle con Eylea.
Otros medicamentos y Eylea
Informe al médico del bebé si este está recibiendo, ha recibido recientemente o pudiera tener que recibir cualquier otro medicamento.
Eylea contiene
- menos de 1 mmol de sodio (23 mg) por unidad de dosis; esto es, esencialmente “exento de sodio”.
- 0,003 mg de polisorbato 20 en cada dosis de 0,01 ml equivalente a 0,3 mg/ml. Los polisorbatos pueden causar reacciones alérgicas. Informe a su médico si tiene cualquier alergia conocida.
- Cómo se le administrará Eylea al bebé
Eylea le será administrado al bebé en los ojos por un médico con experiencia en la administración de inyecciones oculares, en condiciones asépticas (de limpieza y estériles).
La dosis recomendada es de 0,4 mg de aflibercept (0,01 ml).
Eylea se administra en forma de inyección en el interior del ojo del bebé (inyección intravítrea).
Antes de la inyección, el médico del bebé utilizará un lavado ocular desinfectante para limpiar cuidadosamente el ojo del bebé para prevenir una infección. El médico del bebé también le administrará un anestésico local para reducir o prevenir cualquier dolor que pudiera sentir el bebé con la inyección.
El tratamiento se inicia con una inyección única por ojo y puede administrarse en el segundo ojo el mismo día. El médico del bebé controlará el estado del (de los) ojo(s) del bebé. Dependiendo de cómo responda el bebé al tratamiento, el médico del bebé decidirá si se requiere un tratamiento adicional y cuándo debe administrarse. El intervalo de tratamiento entre las dos dosis inyectadas en el mismo ojo debe ser de al menos 4 semanas.
Se presentan instrucciones detalladas para el uso al final de este prospecto en “Cómo preparar y administrar Eylea a bebés prematuros”.
Interrupción del tratamiento con Eylea
Si está considerando interrumpir el tratamiento con Eylea, hable con el médico del bebé en su próxima cita. El médico del bebé le aconsejará y decidirá durante cuánto tiempo se debe tratar al bebé con Eylea.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte al médico del bebé.
- Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos comunicados en más de un bebé nacido prematuramente fueron
- desprendimiento de la capa situada en la parte posterior del ojo(desprendimiento de retina)
- sangrado en la parte posterior del ojo(hemorragia retiniana)
- ojo inyectado en sangredebido a hemorragia procedente de pequeños vasos sanguíneos en las capas externas del ojo (hemorragia conjuntival)
- sangrado en el lugar de inyección(hemorragia en el lugar de inyección)
- aumento de la presión ocular
- hinchazón del párpado(edema palpebral)
A continuación se indican otros efectos adversosque se han observado con Eylea en adultos. Estos efectos adversos también pueden producirse en bebés nacidos prematuramente:
- reacciones alérgicas(hipersensibilidad).
Estas pueden ser graves y requerir que se ponga en contacto con el médico del bebé inmediatamente.
Los efectos adversos que afectan a los ojos debido al procedimiento de inyección pueden ser graves, incluyendo
- ceguera
- infeccióngrave o inflamaciónen el interior del ojo (endoftalmitis)
- desprendimiento, desgarro o hemorragiade la capa sensible a la luz en la parte posterior del ojo (desprendimiento o desgarro de la retina)
- enturbiamiento del cristalino(catarata)
- hemorragia en el ojo(hemorragia vítrea)
- desprendimientode la sustancia similar a un gel que se encuentra en el interior del ojo en contacto con la retina (desprendimiento de vítreo)
- aumento de la presiónen el interior del ojo (aumento de la presión intraocular), ver sección 2.
Estos efectos adversos graves que afectan a los ojos se produjeron en menos de 1 de 1.900 inyecciones durante los ensayos clínicos en adultos.
Es importante identificar y tratar lo antes posible los efectos adversos graves, como una infección en el interior del ojo o un desprendimiento de retina.
Informe inmediatamente al médico del bebé siobserva síntomas en el ojo del bebé después de la inyección, por ejemplo
- enrojecimiento/irritación
- secreción ocular
- hinchazón del párpado
- aumento de la sensibilidad a la luz
A continuación se describen otros efectos adversos observados en adultos.
Lista de los efectos adversos comunicados
A continuación se incluye una lista de los efectos adversos comunicados como posiblemente relacionados con el procedimiento de inyección o con el medicamento. No debe alarmarse, ya que puede que el bebé no experimente ninguno de ellos. Consulte siempre con el médico del bebé acerca de cualquier sospecha de efecto adverso.
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- deterioro de la visión
- sangrado en la parte posterior del ojo (hemorragia retiniana)
- sangre en el ojo debido al sangrado de pequeños vasos sanguíneos en las capas externas del ojo
- dolor ocular
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas):
- desprendimiento o desgarro de una de las capas de la parte posterior del ojo que producen destellos de luz con manchas flotantes que en ocasiones progresa a pérdida de visión (desgarro*/desprendimiento del epitelio pigmentario de la retina, desgarro/desprendimiento de la retina)
- *Efectos adversos que se sabe están asociados a la DMAE exudativa; observados únicamente en pacientes con DMAE exudativa.
- degeneración de la retina que causa alteraciones de la visión
- sangrado en el ojo (hemorragia vítrea)
- ciertas formas de enturbiamiento del cristalino (catarata)
- daños en la capa superficial del globo ocular (la córnea)
- aumento de la presión en el interior del ojo
- manchas en la visión (partículas flotantes)
- desprendimiento de la sustancia similar a un gel que se encuentra en el interior del ojo de la retina (desprendimiento vítreo, que resulta en destellos de luz con manchas flotantes)
- sensación de tener algo dentro del ojo
- aumento de la producción de lágrimas
- hinchazón del párpado
- sangrado en el lugar de inyección
- enrojecimiento del ojo
Efectos adversos poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- reacciones alérgicas (hipersensibilidad)**
- ** Se notificaron reacciones alérgicas como erupción, picor (prurito), ronchas (urticaria) y algunos casos de reacciones alérgicas (anafilácticas/anafilactoides) graves.
- inflamación o infección grave dentro del ojo (endoftalmitis)
- inflamación del iris o de otras partes del ojo (iritis, uveítis, iridociclitis, células flotantes en la cámara anterior)
- sensación anormal en el ojo
- irritación en el párpado
- hinchazón de la capa superficial del globo ocular (córnea)
Efectos adversos raros(pueden afectar hasta 1 de cada 1.000 personas):
- ceguera
- enturbiamiento del cristalino debido a lesión (catarata traumática)
- inflamación de la sustancia similar a un gel que se encuentra en el interior del ojo
- pus en el ojo
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- inflamación de la parte blanca del ojo asociada con enrojecimiento y dolor (escleritis)
El uso de inhibidores del VEGF por vía sistémica, sustancias similares a las contenidas en Eylea, está potencialmente relacionado con el riesgo de formación de coágulos de sangre que bloquean los vasos sanguíneos (eventos tromboembólicos arteriales) que pueden producir un ataque al corazón o una embolia. Hay un riesgo teórico de que pueda producirse este tipo de eventos después de la inyección de Eylea en el ojo.
Al igual que con todas las proteínas terapéuticas, existe la posibilidad de una reacción inmune (formación de anticuerpos) con Eylea.
Si tiene alguna duda sobre algún efecto adverso, pregunte al médico del bebé.
Comunicación de efectos adversos
Si observa cualquier tipo de efecto adverso en el bebé, consulte al médico del bebé, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
- Conservación de Eylea
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en la etiqueta después de “CAD/EXP”. La fecha de caducidad es el último día del mes que se indica.
- Conservar en nevera (entre 2 °C y 8 °C). No congelar.
- El blíster sin abrir puede conservarse fuera de la nevera por debajo de 25 °C durante un máximo de 24 horas.
- Conservar en el embalaje original para protegerlo de la luz.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
- Contenido del envase e información adicional
Composición de Eylea
- El principio activo es: aflibercept. Una jeringa precargada contiene un volumen extraíble de al menos 0,09 ml, equivalente a al menos 3,6 mg de aflibercept. Una jeringa precargada proporciona una dosis única de 0,4 mg de aflibercept en 0,01 ml.
- Los demás componentes son: polisorbato 20 (E 432), dihidrógenofosfato de sodio monohidrato (para el ajuste del pH), hidrogenofosfato de disodio heptahidrato (para el ajuste del pH), cloruro de sodio, sacarosa, agua para preparaciones inyectables.
Ver “Eylea contiene” en la sección 2 para más información.
Aspecto del producto y contenido del envase
Eylea es una solución inyectable (inyectable) en una jeringa precargada. La solución es de incolora a amarillo pálido.
Envase con 1 jeringa precargada.
Titular de la autorización de comercialización
Bayer AG
51368 Leverkusen
Alemania
Responsable de la fabricación
Bayer AG
Müllerstraße 178
13353 Berlin
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België / Belgique / Belgien Bayer SA-NV Tél/Tel: +32-(0)2-535 63 11 | Lietuva UAB Bayer Tel: +370-5-233 68 68 |
| Luxembourg / Luxemburg Bayer SA-NV Tél/Tel: +32-(0)2-535 63 11 |
Ceskárepublika Bayer s.r.o. Tel: +420-266 101 111 | Magyarország Bayer Hungária KFT Tel: +36-1-487 4100 |
Danmark Bayer A/S Tlf: +45-45 235 000 | Malta Alfred Gera and Sons Ltd. Tel: +356-21 44 62 05 |
Deutschland Bayer Vital GmbH Tel: +49-(0)214-30 513 48 | Nederland Bayer B.V. Tel: +31–23-799 1000 |
Eesti Bayer OÜ Tel: +372-655 85 65 | Norge Bayer AS Tlf: +47-23 13 05 00 |
Ελλ?δα Bayer Ελλ?ς ΑΒΕΕ Τηλ: +30-210-618 75 00 | Österreich Bayer Austria Ges. m. b. H. Tel: +43-(0)1-711 460 |
España Bayer Hispania S.L. Tel: +34-93-495 65 00 | Polska Bayer Sp. z o.o. Tel: +48-22-572 35 00 |
France Bayer HealthCare Tél (N° vert): +33-(0)800 87 54 54 | Portugal Bayer Portugal, Lda. Tel: +351-21-416 42 00 |
Hrvatska Bayer d.o.o. Tel: + 385-(0)1-6599 900 | România SC Bayer SRL Tel: +40-(0)21-529 59 00 |
Ireland Bayer Limited Tel: +353-(0)1-216 3300 | Slovenija Bayer d. o. o. Tel: +386-(0)1-58 14 400 |
Ísland Icepharma hf. Sími: +354-540 80 00 | Slovenská republika Bayer, spol. s r.o. Tel: +421-(0)2-59 21 31 11 |
Italia Bayer S.p.A. Tel: +39-02-3978 1 | Suomi/Finland Bayer Oy Puh/Tel: +358-(0)20-78521 |
Κ?προς NOVAGEM Limited Τηλ: +357-22-48 38 58 | Sverige Bayer AB Tel: +46-(0)8-580 223 00 |
Latvija SIA Bayer Tel: +371-67 84 55 63 |
Fecha de la última revisión de esteprospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu.
Si desea información local, escanee aquí para acceder al sitio web https://www.pi.bayer.com/eylea1.
Se incluye un código QR con el enlace al prospecto.
<------------------------------------------------------------------------------------------------------------------
Estainformación está destinada únicamente a profesionales sanitarios:
Cómo preparar y administrar Eylea a recién nacidos pretérmino
La jeringa precargada debe utilizarse para el tratamiento de un solo ojo. La extracción de múltiple dosis de una jeringa precargada puede aumentar el riesgo de contaminación y de posterior infección.
No abrir el blíster precargado estéril fuera de la sala limpia. La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local.
La jeringa precargada contiene más cantidad que la dosis recomendada de 0,4 mg de aflibercept (equivalente a 0,01 ml). Para el tratamiento de recién nacidos pretérmino, se debe usar el dispositivo dosificador pediátrico PICLEO en combinación con la jeringa precargada para administrar una dosis única de 0,4 mg de aflibercept (equivalente a 0,01 ml). Ver la siguiente sección “Instrucciones de uso de la jeringa precargada”.
Antes de la administración, la solución debe inspeccionarse visualmente para detectar la presencia de partículas y/o un cambio de color o cualquier cambio en el aspecto físico. Si observa cualquiera de ellos, no utilice el medicamento.
El blíster sin abrir puede conservarse fuera de la nevera por debajo de 25 °C durante un máximo de 24 horas. Tras la apertura del blíster, proceda bajo condiciones asépticas.
Para la inyección intravítrea debe usarse una aguja de inyección de 30 G x ½ pulgada (1,27 cm).
Instrucciones de uso de la jeringa precargada:
Para preparar la jeringa precargada para la administración a recién nacidos pretérmino, siga los pasos 1 y 2 descritos más adelante y, a continuación, siga las instrucciones de uso incluidas en el envase del dispositivo dosificador pediátrico PICLEO.
- Cuando esté preparado para administrar Eylea, abra la caja y extraiga el bíster esterilizado. Despegue cuidadosamente la lámina del blíster, asegurando la esterilidad de su contenido. Mantenga la jeringa en la bandeja estéril hasta que esté listo para el ensamblaje.
- Utilizando una técnica aséptica, extraer la jeringa del envase del blíster esterilizado.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a EYLEA 40 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 40 mg/mlPrincipio activo: AfliberceptFabricante: Sandoz GmbhRequiere recetaForma farmacéutica: INYECTABLE, 40 mg/mlPrincipio activo: AfliberceptFabricante: Sandoz GmbhRequiere recetaForma farmacéutica: INYECTABLE, 40 mg/mlPrincipio activo: AfliberceptFabricante: Celltrion Healthcare Hungary Kft.Requiere receta
Médicos online para EYLEA 40 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de EYLEA 40 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes