
EPREX 4000 UI/0,4 ml SOLUCION INYECTABLE EN JERINGAS PRECARGADAS

Cómo usar EPREX 4000 UI/0,4 ml SOLUCION INYECTABLE EN JERINGAS PRECARGADAS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
EPREX 1.000 UI/0,5ml solución inyectable en jeringa precargada
EPREX 2.000 UI/0,5ml solución inyectable en jeringa precargada
EPREX 3.000 UI/0,3ml solución inyectable en jeringa precargada
EPREX 4.000 UI/0,4ml solución inyectable en jeringa precargada
EPREX 10.000 UI/ml solución inyectable en jeringa precargada
EPREX 40.000 UI/ml solución inyectable en jeringa precargada
(epoetina alfa)
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento porque contiene información importante para usted.
- Conserve este prospecto ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, enfermero o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas, aunque tengan los mismos signos de enfermedad, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es EPREX y para qué se utiliza
- Qué necesita saber antes de empezar a usar EPREX
- Cómo usar EPREX
- Posibles efectos adversos
- Conservación de EPREX
- Contenido del envase e información adicional
1. Qué es EPREX y para qué se utiliza
EPREX contiene el principio activo epoetina alfa, una proteína que estimula la médula ósea para que produzca más glóbulos rojos, que transportan la hemoglobina (una sustancia que a su vez transporta oxígeno). Epoetina alfa es una copia de la eritropoyetina humana y actúa del mismo modo.
- EPREX se utiliza para tratar la anemia sintomática debida a enfermedades renales
- en niños en hemodiálisis,
- en adultos en hemodiálisis o en diálisis peritoneal,
- en adultos con anemia grave no sometidos aún a diálisis.
Si padece una enfermedad renal y su riñón no elabora suficiente eritropoyetina (necesaria para la formación de glóbulos rojos) puede tener anemia. EPREX se prescribe para estimular su médula ósea a fin de que produzca más glóbulos rojos.
- EPREX se utiliza para tratar la anemia en adultos si recibe quimioterapia por tumores sólidos, linfoma maligno o mieloma múltiple (cáncer de médula ósea) que requiere una transfusión de sangre. EPREX puede reducir la necesidad de transfusiones de sangre en estos pacientes.
- EPREX se utiliza en adultos con anemia moderada que donan parte de su sangre antes de una intervención quirúrgicapara que se les pueda volver a transfundir durante la operación o después de ésta. Como EPREX estimula la producción de glóbulos rojos, los médicos pueden extraer más sangre a estas personas.
- EPREX se emplea en adultos con anemia moderada que van a someterse a cirugía ortopédica mayor electiva(por ejemplo, operaciones de reemplazamiento de prótesis de rodilla o cadera),para reducir la posible necesidad de transfusiones de sangre.
EPREX se utiliza para tratar la anemia en adultos con un trastorno en la médula ósea que causa una alteración grave en la creación de células sanguíneas (síndromes mielodisplásicos).EPREX puede reducir la necesidad de transfusiones de sangre.
2. Qué necesita saber antes de empezar a usar EPREX
No use EPREX
- Si es alérgicoa epoetina alfa o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Si se le ha diagnosticado una Aplasia Pura de Células Rojas(la médula ósea no puede producir suficientes glóbulos rojos) después de un tratamiento previo con cualquier producto que estimula la producción de glóbulos rojos, (incluido EPREX). Consulte la sección 4, Posibles efectos adversos.
- Si tiene la tensión arterial elevaday no está controlada adecuadamente con medicamentos.
- Para estimular la producción de glóbulos rojos (de forma que el médico pueda extraerle más sangre) si no puede recibir transfusiones de su propia sangredurante una intervención quirúrgica o después de ella.
- Si tiene previsto someterse a unacirugía ortopédica mayor electiva(como una prótesis de rodilla o cadera) y:
- tiene una enfermedad cardiaca grave
- tiene problemas graves de las venas y las arterias
- ha tenido recientemente un ataque cardiaco o un accidente cerebrovascular
- no puede tomar medicamentos para diluir la sangre.
Puede que EPREX no sea adecuado para usted. Consulte a su médico. Mientras reciben EPREX, algunas personas necesitan tomar medicamentos para reducir el riesgo de coagulación de la sangre. Si no puede tomar medicamentos que impidan la coagulación de la sangre, no debe utilizar EPREX.
Advertencias y precauciones
Tenga especial cuidado con EPREX
EPREX y otros productos que estimulan la producción de glóbulos rojos pueden aumentar el riesgo de presentar coágulos de sangre en todos los pacientes. Este riesgo puede ser mayor si usted tiene otros factores de riesgopara la aparición de coágulos (por ejemplo, si ha tenido un coágulo de sangre en el pasado o si tiene sobrepeso, si padece diabetes, si tiene una enfermedad del corazón o si está mucho tiempo sin ponerse de pie debido a una operación o enfermedad).Por favor, hable de todo ello con su médico. Su médico le ayudará a decidir si EPREX es adecuado para usted.
Es importante que informe a su médicosi presenta alguna de las circunstancias siguientes. Posiblemente podrá seguir utilizando EPREX, pero consulte antes a su médico.
- Si sabe que padeceo que ha padecido:
- hipertensión arterial;
- convulsioneso crisis epilépticas
- una enfermedad del hígado
- anemia por otras causas
- porfiria (un trastorno raro de la sangre)
- una alergia al látex. La tapa de la aguja de este medicamento contiene goma de látex lo que puede producir reacciones alérgicas graves en personas sensibles al látex. Ver sección 4 para los signos de una reacción alérgica.
- Si es un paciente con insuficiencia renal crónica, y especialmente si no responde adecuadamente al tratamiento con EPREX, su médico revisará su dosis de EPREX ya que, los incrementos repetidos de la dosis de EPREX cuando no se responde al tratamiento puede aumentar el riesgo de sufrir problemas de corazón o de los vasos sanguíneos y puede aumentar el riesgo de sufrir infarto de miocardio, accidente cerebrovascular y muerte.
- Si es un paciente con cáncer, debe saber que los productos que estimulan la producción de glóbulos rojos (como EPREX) pueden actuar como un factor de crecimiento y, en teoría, podrían afectar a la progresión de su cáncer. Dependiendo de su situación personal puede ser preferible una transfusión.Consulte esto a su médico.
- Si es un paciente con cáncer,debe saber que el uso de EPREX puede estar asociado con una menor supervivencia y una mayor tasa de mortalidad en pacientes con cáncer de cabeza y de cuello, con cáncer de mama metastásico, que reciben quimioterapia.
- Se han observado reacciones adversas cutáneas graves, como síndrome de Stevens-Johnson (SSJ) y necrólisis epidérmica tóxica (NET) con la administración de epoetinas.
El SSJ/NET puede aparecer inicialmente como máculas o manchas circulares de color rojo en escarapela, a menudo con ampollas centrales en el tronco. Pueden aparecer también úlceras en la boca, garganta, nariz, genitales y ojos (irritación e hinchazón ocular). Estas erupciones cutáneas graves van precedidas a menudo de fiebre o síntomas de tipo gripal. La erupción cutánea puede progresar a descamación generalizada de la piel y a complicaciones potencialmente mortales.
Si presenta una erupción cutánea grave o alguno de estos otros síntomas cutáneos, deje de tomar EPREX y póngase en contacto con su médico o solicite atención médica de inmediato.
Tenga especial cuidado con otros productos que estimulan la producción de glóbulos rojos:
EPREX pertenece a un grupo de productos que estimulan la producción de glóbulos rojos como lo hace la proteína humana eritropoyetina. Su médico siempre registrará el nombre exacto del producto que usted está utilizando.
Si durante su tratamiento le dan un producto de este grupo distinto a EPREX, comuníqueselo a su médico o farmacéutico antes de utilizarlo.
Otros medicamentos y EPREX
Informe a su médico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Si está tomando un fármaco llamado ciclosporina(que se utiliza, por ejemplo, después de los trasplantes de riñón), su médico puede pedirle análisis de sangre para determinar los niveles de ciclosporina mientras toma EPREX.
Los suplementos de hierro y otros estimulantes de la sangrepueden incrementar la eficacia de EPREX. Su médico decidirá si es adecuado que los tome.
Si acude a un hospital, clínica o médico de atención primaria, informe de que está recibiendo tratamiento con EPREX. Puede afectar a otros tratamientos o a los resultados de algunas pruebas.
Embarazo y lactancia
Es importante que avise a su médicosi algo de lo siguiente le aplica a usted. Usted probablemente va a poder seguir utilizando EPREX, pero háblelo primero con su médico.
- Si está embarazada,o piensa que pudiera estarlo.
- Si está dando el pecho.
EPREX contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
EPREX contiene polisorbato 80
Este medicamento contiene un máximo de 0,30 mg de polisorbato 80 en cada jeringa, equivalente a una concentración de 0,30 mg/ml. Los polisorbatos pueden causar reacciones alérgicas. Informe a su médico si usted o su hijo tiene cualquier alergia conocida.
3. Cómo usar EPREX
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
Su médico le ha hecho análisis de sangrey ha decidido que necesita EPREX.
EPREX se puede administrar mediante inyección:
- Tantoen una vena o un tubo introducido en una vena (intravenosa)
- Comobajo la piel (subcutánea).
Su médico decidirá cómo hay que administrar EPREX. Habitualmente, las inyecciones las pondrá un médico, un enfermero u otro profesional sanitario. Algunas personas, dependiendo del motivo por el que necesiten EPREX, luego pueden aprender a ponerse la inyección bajo la piel: consulte Instrucciones para autoadministrarse EPREX.
EPREX no debe usarse:
- después de la fecha de caducidad de la etiqueta y del envase exterior
- si sabe o sospecha que puede haber sido congelado accidentalmente, o
- si se ha averiado la nevera.
La dosis de EPREX que recibirá se basa en su peso corporal expresado en kilogramos. La causa de su anemia también es un factor que su médico tendrá en cuenta al decidir la dosis correcta.
Su médico vigilará su tensión arterialde forma regular mientras utilice EPREX.
Personas con enfermedad renal
- Su médico mantendrá sus niveles de hemoglobina entre 10 y 12 gramos/decilitro ya que los niveles altos de hemoglobina pueden aumentar el riesgo de coágulos de sangre y muerte. En niños se deben mantener los niveles de hemoglobina entre 9,5 y 11 gramos/decilitro.
- La dosis inicial habitualde EPREX en adultos y niños es de 50 Unidades Internacionales (UI) por kilogramo (/kg) de peso corporal tres veces a la semana.
- En los pacientes sometidos a diálisis peritoneal, EPREX se puede administrar dos veces a la semana.
- En los adultos y niños EPREX se administra en forma de inyección en una vena o en un tubo introducido en una vena. Cuando este acceso (vena o tubo) no está fácilmente disponible, su médico puede decidir que EPREX se inyecte bajo la piel (inyección subcutánea). Este caso también aplica a pacientes en diálisis y a los pacientes que todavía no están en diálisis.
- Su médico solicitará análisis de sangre de forma regular para vigilar la respuesta de su anemia y poder ajustar la dosis, habitualmente con una frecuencia no superior a cada cuatro semanas. Se debe evitar un incremento de hemoglobina superior a 2 gramos/decilitro en un periodo de cuatro semanas.
- Después de corregir la anemia, su médico continuará vigilando su sangre de forma regular. Es posible que haga nuevos ajustes de la dosis y de la frecuencia de administración de EPREX para mantener su respuesta al tratamiento. Su médico utilizará la menor dosis efectiva para controlar los síntomas de su anemia.
- Si usted no responde adecuadamente a EPREX, su médico revisará su dosis y le informará en el caso de que sea necesario cambiar sus dosis de EPREX.
- Si usted sigue un intervalo de dosis de EPREX más amplio (mayor de una vez a la semana) puede que no mantenga niveles de hemoglobina adecuados y puede necesitar un aumento de la dosis o de la frecuencia de administración de EPREX.
- Cabe la posibilidad de que reciba suplementos de hierro antes y durante el tratamiento con EPREX para que sea más eficaz.
- Si está recibiendo diálisis en el momento de iniciar el tratamiento con EPREX, puede ser necesario ajustar su régimen de diálisis. Todo esto lo decidirá su médico.
Adultos que reciben quimioterapia
- Su médico puede iniciar el tratamiento con EPREX si su hemoglobina es 10 gramos/ decilitro o menor.
- Su médico mantendrá sus niveles de hemoglobina entre 10 y 12 gramos/decilitro ya que los niveles altos de hemoglobina pueden aumentar el riesgo de coágulos de sangre y muerte.
- La dosis inicial es de tanto150 UI por kilogramo de peso corporal tres veces a la semana comode 450 UI por kilogramo de peso corporal una vez a la semana.
- EPREX se administra mediante inyección subcutánea.
- Su médico solicitará análisis de sangre y puede ajustar la dosis, dependiendo de la respuesta de su anemia al tratamiento con EPREX.
- Cabe la posibilidad de que reciba suplementos de hierro antes y durante el tratamiento con EPREX para que sea más eficaz.
- Normalmente continuará el tratamiento con EPREX durante un mes después de que termine la quimioterapia.
Adultos que donan sangre para autotransfusión
- La dosis habituales de 600 UI por kilogramo de peso corporal dos veces a la semana.
- EPREX se administra como inyección intravenosa inmediatamente después de que haya donado sangre durante las 3 semanas previas a la intervención quirúrgica.
- Cabe la posibilidad de que reciba suplementos de hierro antes y durante el tratamiento con EPREX para que sea más eficaz.
Pacientes que van a someterse a una cirugía ortopédica mayor
- La dosis recomendadaes de 600 UI por kilogramo de peso corporal una vez a la semana.
- EPREX se administra como inyección subcutánea cada semana durante las tres semanas previas a la intervención quirúrgica y el día de la operación.
- Si por motivos médicos es necesario reducir el intervalo hasta la operación, recibirá una dosis diaria de 300 UI/kg durante un periodo de hasta diez días antes de la intervención, el día de la operación y en los cuatro días inmediatamente posteriores.
- El tratamiento se interrumpirá si los análisis de sangre indican que los valores de hemoglobina son demasiado elevados antes de la operación.
- Cabe la posibilidad de que reciba suplementos de hierro antes y durante el tratamiento con EPREX para que sea más eficaz.
Adultos con síndrome mielodisplásico
- Su médico puede iniciar el tratamiento con EPREX si su hemoglobina es menor o igual a 10 gramos/ decilitro. El objetivo del tratamiento es mantener sus niveles de hemoglobina entre 10 y 12 gramos/decilitro ya que niveles más altos de hemoglobina pueden aumentar el riesgo de coágulos de sangre y muerte.
- EPREX se administra como inyección subcutánea.
- La dosis inicial es de 450 UI por kilogramo de peso corporal una vez a la semana.
- Su médico solicitará análisis de sangre y puede ajustar la dosis, dependiendo de la respuesta de su anemia al tratamiento con EPREX.
Instrucciones para la autoinyección de EPREX
Cuando comienza el tratamiento, EPREX suele ser administrado por personal médico o de enfermería. Más tarde, su médico puede sugerir que usted o un cuidador aprendan a inyectar EPREX bajo la piel (por vía subcutánea).
- No intente inyectarse el medicamento salvo que un médico o enfermero le haya enseñado.
- Utilice siempre EPREX siguiendo las instrucciones exactas de su médico o enfermero.
- Use EPREX sólo si se ha conservado correctamente – Ver sección 5,Conservación de EPREX.
- Antes del uso, espere a que la jeringa de EPREX alcance la temperatura ambiente.Esto requiere habitualmente 15‑30 minutos.
Extraiga sólo una dosis de EPREX de cada jeringa.
Si se inyecta EPREX debajo de la piel (inyección subcutánea), la cantidad administrada en cada inyección normalmente no es mayor de un mililitro (1 ml).
EPREX se administra solo y no se mezcla con otros líquidos para inyección.
No agite las jeringas de EPREX.La agitación enérgica prolongada puede deteriorar el producto. No utilice el producto si se ha agitado enérgicamente.
Cómo autoinyectarse por vía subcutánea utilizando una jeringa precargada:
Las jeringas precargadas disponen de un dispositivo de protección de la aguja PROTECS™ que ayuda a prevenir pinchazos tras su utilización. Esto viene indicado en la caja.
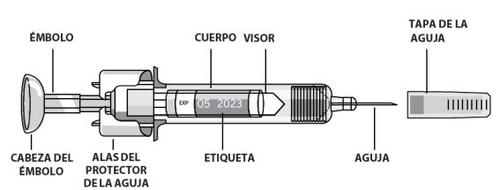
- Saque una jeringa del frigorífico.Es necesario que el líquido alcance la temperatura ambiente. No retire la tapa de la aguja de la jeringa mientras alcanza la temperatura ambiente.
- Compruebe la jeringa,para asegurarse de que la dosis es correcta, no se ha sobrepasado la fecha de caducidad, no está deteriorada y el líquido está transparente y no se ha congelado.
- Retire la parte despegable de la etiqueta de la jeringa. Si usted no puede ver las marcas de graduación a través del visor, sujete el cuerpo de la jeringa y gire suvamente la jeringa por la tapa de la aguja para alinear las marcas de graduación con el visor.
- Elija el sitio de inyección.Los lugares más adecuados son la parte superior del muslo y alrededor de la tripa (abdomen) pero lejos del ombligo. Cambie de sitio de un día a otro.
- Lávese las manos.Aplique una gasa con antiséptico sobre el lugar de la inyecciónpara desinfectarlo.
- Sujete la jeringa precargada por el cuerpo de la jeringa con la aguja tapada apuntando hacia arriba.
- No la sujete por la cabeza del émbolo, el émbolo, las alas del protector de la aguja ni de la tapa de la aguja.
- No tire del émbolo en ningún momento
- No retire la tapa de la aguja de la jeringa precargada hasta que esté preparado para inyectarse EPREX.
- Retire la tapa de la jeringasosteniendo el cuerpo y tirando de la tapa de la aguja con precaución, sin girarla. No toque la aguja ni agite la jeringa.
- Elimine la burbuja de aire sujetando la jeringa con la aguja apuntando hacia arriba y presione suavemente el émbolo hasta que una gota de líquido salga por la punta de la aguja.
- En caso de que solo necesite una dosis parcial de la jeringa tal y como le ha indicado su médico, presione el émbolo hasta la marca de graduación numerada deseada para eliminar el líquido que no necesite antes de la inyección.
- No toque las alas del protector de la aguja para evitar que la aguja quede tapada prematuramente con el protector de la aguja.
- Sostenga un pliegue de pielentre los dedos pulgar e índice. No lo apriete.
- Introduzca toda la aguja.Su médico o enfermero le habrán enseñado a hacerlo.
- Empuje el émbolo con su dedo pulgar hasta el final para inyectar la cantidad total de líquido. Hágalo de forma lenta y uniforme, manteniendo el pliegue de piel pellizcado. El protector de la agujaPROTECS™no se activará a menos que se administre la dosis entera. Debe oír uncliccuando el protector de la aguja PROTECS™se haya activado.
- Cuando haya presionado el émbolo hasta el tope, retire la aguja y suelte el pliegue de piel.
- Quite lentamente su dedo pulgar del émbolopara permitir que la jeringa se desplace hasta que toda la aguja sea cubierta por el protector de la aguja PROTECS™.
- Cuando retire la aguja de su piel, puede sangrar un poco en el lugar de la inyección. Esto es normal. Puede presionarel lugar de la inyección durante varios segundos con una gasa con antisépticodespués de la inyección.
- Deposite la jeringa usadaen un recipiente seguro; consulte la sección 5, Conservación de EPREX.
Si usa más EPREX del que debe
Informe inmediatamente a su médico o enfermero si considera que se ha inyectado una cantidad excesiva de EPREX. Es improbable que aparezcan efectos adversos como consecuencia de una sobredosis de EPREX.
Si olvidó usar EPREX
Póngase la siguiente inyección lo antes posible. Si faltan menos de 24 horas hasta la siguiente inyección, olvide la que se ha saltado y continúe con su calendario normal. No duplique las inyecciones para compensar las dosis olvidadas.
Si usted es un paciente con Hepatitis C y recibe interferón y ribavirina
Debe consultar con su médico ya que la combinación de epoetina alfa con interferón y ribavirina ha dado lugar a casos raros de pérdida de eficacia y al desarrollo de un trastorno llamado aplasia pura de células rojas (APCR), una forma grave de anemia. EPREX no está autorizado para el manejo de la anemia asociada a hepatitis C.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Informe a su médico o enfermero inmediatamentesi detecta alguno de los efectos citados en esta lista.
Se han observado erupciones cutáneas graves, como síndrome de Stevens-Johnson y necrólisis epidérmica tóxica, con la administración de epoetinas. Estas reacciones pueden aparecer como máculas o manchas circulares de color rojo en escarapela, a menudo con ampollas centrales en el tronco, descamación de la piel y úlceras en la boca, garganta, nariz, genitales y ojos y pueden ir precedidas de fiebre y síntomas de tipo gripal. Deje de usar EPREX si presenta estos síntomas y póngase en contacto con su médico o solicite atención médica de inmediato. Ver también la sección 2.
Efectos adversos muy frecuentes
Éstos pueden afectar a más de 1 de cada 10 personas
- Diarrea
- Malestar de estómago
- Vómitos
- Fiebre
- Se ha notificado congestión del tracto respiratorio, como nariz taponada y dolor de garganta, en pacientes con enfermedad renal no sometidos todavía a diálisis.
Efectos adversos frecuentes
Éstos pueden afectar hasta a 1 de cada 10 personas
- Aumento de la presión sanguínea. Dolores de cabezaespecialmente si son de tipo migrañoso, punzante y repentinos, sensación de confusión o convulsionespueden ser signos de un aumento repentino de la tensión arterial. Esto requiere tratamiento urgente. La tensión arterial elevada puede requerir tratamiento con medicamentos (o el ajuste de algunos medicamentos que ya esté tomando para la hipertensión).
- Coágulos de sangre(incluyendo trombosis venosa profunda y embolia) que pueden necesitar tratamiento urgente. Los síntomas que puede presentar son doloren el pecho, dificultad para respirar, e inflamación dolorosay enrojecimiento, normalmentede una pierna.
- Tos
- Erupciones cutáneas, que pueden ser manifestaciones de una reacción alérgica.
- Dolor de huesos o músculos
- Síntomas de tipo gripal, como dolor de cabeza, pinchazos y dolores en las articulaciones, sensación de debilidad, escalofríos, cansancio y mareo. Pueden ser más frecuentes al inicio del tratamiento. Si presenta estos síntomas durante la inyección intravenosa, una administración más lenta de la inyección puede ayudar a evitar que ocurran.
- Enrojecimiento, ardor y dolor en el lugar de la inyección
- Hinchazón de los tobillos, los pies o los dedos
- Dolor de brazo o pierna
Efectos adversos poco frecuentes
Éstos pueden afectar hasta a 1 de cada 100 personas
- Niveles altos de potasio en la sangreque pueden causar anomalías del ritmo del corazón (se trata de un efecto adverso muy frecuente en los pacientes sometidos a diálisis).
- Crisis epilépticas
- Congestión nasal o de las vías respiratorias
- Reacción alérgica
- Habones
Efectos adversos raros
Éstos pueden afectar hasta a 1 de cada 1.000 personas
- Síntomas de aplasia pura de células rojas (APCR).
La APCR es la incapacidad para producir suficientes glóbulos rojos en la médula ósea. La APCR puede provocar una anemia repentina y grave. Los síntomas son:
- cansancio inusual,
- sensación de mareo,
- dificultad para respirar.
Se han notificado casos muy raros de APCR principalmente en pacientes con enfermedad renal después de meses o años de tratamiento con EPREX y otros productos que estimulan la producción de glóbulos rojos.
- Se puede producir un aumento de la cantidad de unas células sanguíneas pequeñas (llamadas plaquetas) que normalmente participan en la formación de los coágulos de sangre, especialmente cuando se inicia el tratamiento. Su médico lo comprobará.
- Reacciones alérgicas graves que pueden incluir:
- cara, labios, boca, lengua o garganta hinchada
- dificultad para tragar o respirar
- erupción con picor (habones)
- Trastorno que afecta a la sangre que puede causar dolor, orina con color oscuro o aumento de la sensibilidad en la piel a la luz solar (porfiria).
Si está recibiendo hemodiálisis:
- Se pueden formar coágulos de sangre(trombosis) en la fístula de la diálisis (shunt). Esto es más frecuente si tiene la tensión arterial baja o si su fístula presenta complicaciones.
- También se pueden formar coágulos de sangreen su sistema de hemodiálisis. Su médico puede decidir aumentar su dosis de heparina durante la diálisis.
Si padece alguno de estos efectos o si observa cualquier otro efecto mientras esté en tratamiento con EPREX, comuníqueselo inmediatamente a su médico o enfermero.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de EPREX
Mantener este medicamento fuera de la vista y el alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en la caja después de las letras CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 °C y 8 °C). Usted puede sacar EPREX de la nevera y mantenerlo a temperatura ambiente (hasta 25ºC) durante 3 días. Una vez que la jeringa ha sido sacada de la nevera y ha alcanzado la temperatura ambiente (hasta 25ºC) debe ser utilizado dentro de los 3 días siguientes o sino deberá ser desechado.
No congelar ni agitar.
Conservar en el envase original para protegerlo de la luz.
No utilice este medicamento si observa que el precinto está roto o si el líquido presenta coloración o se pueden observar partículas en suspensión. Si observa alguna de estas cosas, deseche el medicamento.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de EPREX:
El principio activo es:epoetina alfa (ver cantidades en la tabla).
Los demás componentes son:polisorbato 80 (E 433), cloruro de sodio, dihidrógeno fosfato de sodio monobásico dihidratado, fosfato de sodio dibásico dihidratado, glicina y agua para preparaciones inyectables.
Aspecto de EPREX y contenido del envase
EPREX se presenta en forma de solución inyectable en jeringa precargada. Las jeringas precargadas llevan acoplado un dispositivo de protección de la aguja PROTECS™ (ver tabla a continuación). EPREX es una solución transparente e incolora.
Presentación | Presentaciones equivalentes en cantidad/volumen para cada concentración | Cantidad de epoetina alfa |
Envase con 6 jeringas precargadas con un dispositivo de protección de la aguja PROTECS™ | 1.000 UI/0,5 mililitros 2.000 UI/0,5 mililitros 3.000 UI/0,3 mililitros 4.000 UI/0,4 mililitros 5.000 UI/0,5 mililitros 6.000 UI/0,6 mililitros 8.000 UI/0,8 mililitros 10.000 UI/1 mililitros | 8,4 microgramos 16,8 microgramos 25,2 microgramos 33,6 microgramos 42,0 microgramos 50,4 microgramos 67,2 microgramos 84,0 microgramos |
Envase con 1 jeringa precargada con un dispositivo de protección de la aguja PROTECS™ | 20.000 UI/0,5 mililitros 30.000 UI/0,75 mililitros 40.000 UI/1 mililitro | 168 microgramos 252 microgramos 336 microgramos |
Envase con 4 jeringas precargadas con un dispositivo de protección de la aguja PROTECS™ | 20.000 UI/0,5 mililitros 30.000 UI/0,75 mililitros 40.000 UI/1 mililitro | 168 microgramos 252 microgramos 336 microgramos |
Envase con 6 jeringas precargadas con un dispositivo de protección de la aguja PROTECS™ | 20.000 UI/0,5 mililitros 30.000 UI/0,75 mililitros 40.000 UI/1 mililitro | 168 microgramos 252 microgramos 336 microgramos |
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización:
Janssen-Cilag, S.A.
Paseo del Club Deportivo 1, Edificio 16
28223 Pozuelo de Alarcón (Madrid)
España
Responsable de la fabricación:
Janssen Biologics BV
Einsteinweg 101
2333 CB
Leiden
Países Bajos
Este medicamento está autorizado en los estados miembros del EEE y en el Reino Unido (Irlanda del Norte) con los siguientes nombres:
Austria: ERYPO?
Bélgica: EPREX?
Alemania: ERYPO?
Grecia: EPREX?
Francia: EPREX?
Italia: EPREX?
Luxemburgo: EPREX?
Países Bajos: EPREX?
Portugal: EPREX?
España: EPREX?
Reino Unido (Irlanda del Norte): EPREX?
Este prospecto ha sido aprobado en:enero 2025.
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/).
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a EPREX 4000 UI/0,4 ml SOLUCION INYECTABLE EN JERINGAS PRECARGADASForma farmacéutica: INYECTABLE, 20000 UIPrincipio activo: erythropoietinFabricante: Sandoz GmbhRequiere recetaForma farmacéutica: INYECTABLE, 20 kilo UIPrincipio activo: erythropoietinFabricante: Sandoz GmbhRequiere recetaForma farmacéutica: INYECTABLE, 40.000 UI/ml de epoyetina alfaPrincipio activo: erythropoietinFabricante: Sandoz GmbhRequiere receta
Médicos online para EPREX 4000 UI/0,4 ml SOLUCION INYECTABLE EN JERINGAS PRECARGADAS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de EPREX 4000 UI/0,4 ml SOLUCION INYECTABLE EN JERINGAS PRECARGADAS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















