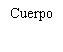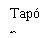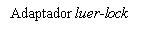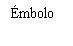ENGERIX- B JUNIOR 10 microgramos/0,5 ml, SUSPENSIÓN INYECTABLE EN JERINGA PRECARGADA


Cómo usar ENGERIX- B JUNIOR 10 microgramos/0,5 ml, SUSPENSIÓN INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Engerix-B Junior 10 microgramos/0,5 ml suspensión inyectable en jeringa precargada
Vacuna de hepatitis B (ADNr, adsorbida) (VHB)
Lea todo el prospecto detenidamente antes de que usted/su hijo empiece a recibir esta vacuna, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Esta vacuna se le ha recetado solamente a usted/su hijo, y no debe dársela a otras personas.
- Si ustedexperimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
A lo largo de este prospecto, cualquier referencia a “usted” también puede significar “su hijo”.
Contenido del prospecto
- Qué es Engerix-B Junior y para qué se utiliza
- Qué necesita saber antes de que usted reciba Engerix-B Junior
- Cómo se administra Engerix-B Junior
- Posibles efectos adversos
- Conservación de Engerix-B Junior
- Contenido del envase e información adicional
1. Qué es Engerix-B Junior y para qué se utiliza
Engerix‑B Junior es una vacuna que se utiliza para proteger frente a la infección por hepatitis B. También puede ayudar a proteger frente a la infección por hepatitis D.
Esta vacuna puede administrarse a recién nacidos, niños y adolescentes de hasta 15 años de edad inclusive.
La hepatitis B es una enfermedad infecciosa del hígado causada por un virus. Algunas personas tienen el virus de la hepatitis B en su organismo, pero no pueden eliminarlo. Estas personas pueden continuar infectando a otras y se les conoce como portadoras. La propagación de la enfermedad se produce cuando el virus entra en el organismo tras el contacto con fluidos corporales, casi siempre sangre, de una persona infectada.
Si una madre es portadora del virus, puede transmitir el virus a su hijo durante el parto. Asimismo, es posible contraer el virus de un portador mediante, por ejemplo, la práctica de sexo sin protección, el uso compartido de jeringas inyectables o el tratamiento con un equipo médico que no haya sido esterilizado adecuadamente.
Los principales signos de la enfermedad incluyen: dolor de cabeza, fiebre, malestar (náuseas) e ictericia (la piel y los ojos adquieren un color amarillento), aunque en aproximadamente tres de cada 10 pacientes no hay signos de enfermedad. De estos infectados con hepatitis B, uno de cada 10 adultos y hasta nueve de cada 10 niños se convertirán en portadores del virus y es probable que desarrollen daños hepáticos graves y en algunos casos, cáncer de hígado.
Cómo funciona Engerix-B Junior
Engerix-B Junior contiene una pequeña cantidad de la cubierta externa del virus de la hepatitis B. Esta cubierta externa no es infecciosa y no puede causar la enfermedad.
- Cuando se le administra la vacuna, ésta activará el sistema inmunitario del organismo para prepararlo para protegerse frente a estos virus en el futuro.
- Engerix-B Junior no le protegerá si ya ha contraído el virus de la hepatitis B.
- Engerix-B Junior sólo puede ayudarle a protegerse frente a la infección por el virus de la hepatitis B.
2. Qué necesita saber antes de que usted reciba Engerix-B Junior
No se debe administrar Engerix-B Junior:
- Si usted es alérgico (hipersensible) a Engerix-B Junior o a cualquiera de los demás componentes de esta vacuna (incluidos en la sección 6).
- Si usted tiene fiebre (temperatura corporal alta).
Engerix-B Junior no debe administrarse si cualquiera de las circunstancias anteriores le afecta a usted. Si no está seguro, hable con su médico o farmacéutico antes de recibir Engerix-B Junior. Informe a su médico o farmacéutico si usted tiene alguna alergia o si usted ha tenido alguna vez problemas de salud después de haber recibido una vacuna.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de que recibir Engerix-B Junior si usted:
- Está en diálisis por un problema renal o si tiene una enfermedad que pueda afectar a su sistema inmunitario.
Las personas que necesitan diálisis, que tienen problemas hepáticos crónicos, que son portadoras de hepatitis C o que son VIH positivos también pueden recibir Engerix-B Junior. Esto se debe a que las infecciones por hepatitis B pueden ser graves en estos pacientes. Encontrará más información acerca de problemas renales y diálisis en la sección 3.
Si no está seguro de si alguna de las anteriores situaciones le afecta, consulte a su médico antes de recibir Engerix-B Junior.
Antes o después de cualquier inyección, podría producirse un desmayo (especialmente en los adolescentes), por lo que debe informar a su médico o enfermero si usted se ha desmayado en anteriores ocasiones tras la administración de una inyección.
Al igual que otras vacunas, puede que Engerix-B Junior no le proteja completamente a usted frente a la hepatitis B. Un cúmulo de factores tales como la edad avanzada, el sexo, el sobrepeso, ser fumador y algunos problemas crónicos reducen la respuesta inmunitaria a la vacuna. Si algo de esto le afecta, su médico puede decidir realizarle un análisis de sangre o administrarle dosis adicionales de Engerix-B Junior para asegurarse de que está protegido.
Uso de Engerix-B Juniorcon otros medicamentos
Comunique a su médico o farmacéutico siusted está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Engerix-B Junior se puede administrar al mismo tiempo que la mayoría de las vacunas habituales. Su médico se asegurará de que las vacunas se inyectan por separado y en distintos lugares del cuerpo.
Embarazo y lactancia
- Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
Es poco probable que Engerix-B Junior afecte a su capacidad para conducir o utilizar máquinas. Sin embargo, no conduzca o maneje máquinas si se siente mal.
Engerix-B Junior contiene sodio
Este medicamento contiene menos de 23 mg (1 mmol) de sodio por dosis, por lo que se considera esencialmente “exento de sodio”.
3. Cómo se administra Engerix-B Junior
Cómo se administra su vacuna
El médico le administrará la dosis recomendada de Engerix-B Junior.
Engerix-B Junior se administrará:
- como una inyección en el músculo de la parte superior del brazo en niños y adolescentes
- como una inyección en el muslo en recién nacidos y niños pequeños
- como una inyección bajo la piel si le aparecen moratones con facilidad o si tiene problemas de coagulación.
Cuánto se administra
Se le administrará una serie de inyecciones de Engerix-B Junior. Una vez que haya terminado la pauta de inyecciones usted puede esperar protección a largo plazo frente a la hepatitis B.
- Los recién nacidos, niños y adolescentes hasta los 15 años de edad inclusive recibirán normalmente la vacuna de 10 microgramos/0,5 ml.
Existen varias alternativas de administración de Engerix-B Junior. Su médico elegirá la pauta más adecuada para usted.
Esquema 1 – para recién nacidos, niños y adolescentes hasta los 15 años de edad inclusive
Primera inyección - ahora
Segunda inyección - 1 mes después de la primera inyección
Tercera inyección - 6 meses después de la primera inyección
Esquema 2 – para recién nacidos, niños y adolescentes hasta los 15 años de edad inclusive
Primera inyección - ahora
Segunda inyección - 1 mes después de la primera inyección
Tercera inyección - 2 meses después de la primera inyección
Cuarta inyección - 12 meses después de la primera inyección
- En recién nacidos el esquema 2 permitirá que Engerix-B Junior se administre al mismo tiempo que otras vacunas habituales de la infancia.
- Este esquema también puede usarse en el caso de que se vacune debido a una exposición reciente a la hepatitis B, ya que le proporcionará una protección más rápida.
Es muy importante que acuda a recibir sus inyecciones con la periodicidad recomendada. Si tiene cualquier pregunta acerca de la cantidad de vacuna que se le va a administrar, consulte con su médico.
Vacunación y parto
Si tiene hepatitis B y acaba de dar a luz, puede utilizarse el esquema 1 ó 2 para vacunar a su hijo.
- Su médico también decidirá si administrar a su hijo inmunoglobulinas de hepatitis B (anticuerpos humanos) en el momento de la primera inyección. Esto ayudará a proteger a su hijo de la hepatitis B. Se administrarán en otro lugar de inyección.
Problemas renales y diálisis
Si su hijo tiene un problema renal o recibe diálisis, su médico puede decidir hacerle un análisis de sangre o administrarle dosis extra de la vacuna para asegurarse de que su hijo está protegido.
4. Posibles efectos adversos
Al igual que todas las vacunas, esta vacuna puede producir efectos adversos, aunque no todas las personas los sufran. Los siguientes efectos adversos pueden ocurrir con esta vacuna:
Reacciones alérgicas
Si tiene una reacción alérgica, acuda a su médico inmediatamente. Los signos pueden incluir:
- hinchazón de la cara
- tensión arterial baja
- respiración dificultosa
- la piel se vuelve azul
- pérdida de conocimiento.
Normalmente estos signos comienzan muy poco después de que le administren la inyección. Acuda a un médico inmediatamente si le ocurren después de dejar la consulta.
Otros efectos adversos incluyen:
Muy frecuentes(estas pueden ocurrir con más de 1 de cada 10 dosis de la vacuna): dolor de cabeza, dolor y enrojecimiento en el lugar de la inyección, sensación de cansancio, irritabilidad.
Frecuentes(estas pueden ocurrir hasta con 1 de cada 10 dosis de la vacuna): adormecimiento, náuseas o vómitos, diarrea o dolor abdominal, pérdida de apetito, fiebre (temperatura corporal alta), sensación de malestar general, hinchazón en el lugar de la inyección, reacciones en el lugar de la inyección, como induración.
Poco frecuentes(estas pueden ocurrir hasta con 1 de cada 100 dosis de la vacuna): mareo, dolor muscular, síntomas parecidos a los de la gripe.
Raros(estas pueden ocurrir hasta con 1 de cada 1.000 dosis de la vacuna): glándulas hinchadas, habones, erupción cutánea y prurito, dolor articular, hormigueo.
Los efectos adversos notificados durante la comercialización de Engerix-B Junior son: fácil aparición de moratones e incapacidad para detener un sangrado si se hace un corte, tensión arterial baja, inflamación de los vasos sanguíneos, hinchazón repentina de la cara alrededor de la boca y de la zona de la garganta (edema angioneurótico), incapacidad para mover los músculos (parálisis), inflamación de los nervios (neuritis) que puede causar pérdida de sensibilidad o entumecimiento, incluyendo una inflamación temporal de los nervios, causando dolor, debilidad y parálisis en las extremidades y a menudo progresando al pecho y a la cara (síndrome de Guillain-Barré), una enfermedad de los nervios del ojo (neuritis óptica) y esclerosis múltiple, problemas para mover los brazos o piernas (neuropatía), inflamación del cerebro (encefalitis), enfermedad degenerativa del cerebro (encefalopatía), infección alrededor del cerebro (meningitis), ataques (convulsiones), pérdida de sensibilidad de la piel al dolor o al tacto (hipoestesia), bultos de color púrpura o púrpura-rojizo en la piel (líquen plano), puntos rojos o de color púrpura en la piel, dolor y rigidez en las articulaciones (artritis), debilidad de los músculos.
En recién nacidos muy prematuramente (a las 28 semanas de gestación o antes) los periodos entre respiraciones pueden ser más largos de lo normal durante los 2-3 días posteriores a la vacunación.
Comunicación de efectos adversos
Si usted experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano, https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Engerix-B Junior
- Mantener esta vacuna fuera de la vista y del alcance de los niños.
- No utilice esta vacuna después de la fecha de caducidad que aparece en la etiqueta y el envase, después de CAD. La fecha de caducidad es el último día del mes que se indica.
- Conservar en nevera (entre 2 ºC y 8 ºC).
- No congelar.
- Conservar en el embalaje original para protegerla de la luz.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Engerix-B Junior
- El principio activo es la cubierta externa del virus de la hepatitis B. Cada dosis contiene 10 microgramos/0,5 ml de proteína compuesta por esta cubierta externa adsorbida en hidróxido de aluminio hidratado.
- Los demás componentes son cloruro de sodio, fosfato de sodio dihidrato, fosfato de sodio dibásico y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Engerix-B Junior es un líquido inyectable blanco turbio.
Engerix-B Junior (10 microgramos/0,5 ml) está disponible en jeringa precargada de 1 dosis con o sin agujas separadas; tamaños de envase de 1 y 10.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
GlaxoSmithKline, S.A.
P.T.M. C/ Severo Ochoa, 2
28760 Tres Cantos (Madrid)
Tel.: +34 900 202 700
Responsable de la fabricación
GlaxoSmithKline Biologicals s.a., Rue de l’Institut 89, B-1330 Rixensart, Bélgica
o
SmithKline Beecham, S.A., Carretera de Ajalvir. Km. 2,5.- 28806 Alcalá de Henares. Madrid
Este medicamento está autorizado en los Estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria, Dinamarca, Finlandia, Islandia, Noruega, Suecia: Engerix- B
Bélgica, Luxemburgo: Engerix B Junior
Francia, Irlanda, Italia: Engerix B-10
Alemania: Engerix- B Niños
Grecia: Engerix
España, Holanda: Engerix-B Junior
Portugal: Engerix B
Fecha de la última revisión de este prospecto:02/2024
Otras fuentes de información
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
---------------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
Una vez almacenada, el contenido puede presentarse como un depósito blanco fino con un sobrenadante claro incoloro. Tras la agitación, la vacuna es ligeramente opaca.
Antes de utilizar la vacuna se debe inspeccionar visualmente para detectar partículas extrañas y/o un aspecto físico anornal. No administrar la vacuna si observa cualquiera de los dos.
Debe extraerse todo el contenido del envase monodosis y debe utilizarse inmediatamente tras la extracción.
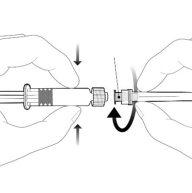
Instrucciones para la jeringa precargada
| Sostenga la jeringa por el cuerpo, no por el émbolo. Desenrosque el tapón de la jeringa girándola en sentido contrario a las agujas del reloj. |
| Para insertar la aguja, conecte la base al adaptador luer-locky gírelo un cuarto de vuelta en el sentido de las agujas del reloj hasta que sienta que se bloquea. No saque el émbolo de la jeringa del cuerpo. Si esto ocurre, no administre la vacuna. |
Eliminación de residuos
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él, se realizará de acuerdo con la normativa local.
- País de registro
- Precio medio en farmacia10.27 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ENGERIX- B JUNIOR 10 microgramos/0,5 ml, SUSPENSIÓN INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 20 mcg ANTIGENO SUPERFICIE HEPATITIS B/ mlPrincipio activo: hepatitis B, purified antigenFabricante: Glaxosmithkline S.A.Requiere recetaForma farmacéutica: INYECTABLE, 20 µgPrincipio activo: hepatitis B, purified antigenFabricante: Glaxosmithkline BiologicalsRequiere recetaForma farmacéutica: INYECTABLE, 20 µgPrincipio activo: hepatitis B, purified antigenFabricante: Glaxosmithkline BiologicalsRequiere receta
Médicos online para ENGERIX- B JUNIOR 10 microgramos/0,5 ml, SUSPENSIÓN INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ENGERIX- B JUNIOR 10 microgramos/0,5 ml, SUSPENSIÓN INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes