ЭЛИГАРД СЕМЕСТРАЛЬ 45 мг ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА

Спросите врача о рецепте на ЭЛИГАРД СЕМЕСТРАЛЬ 45 мг ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА

Инструкция по применению ЭЛИГАРД СЕМЕСТРАЛЬ 45 мг ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА
Введение
Прошпект: информация для пользователя
Элигард полугодовой 45 мг
порошок и растворитель для раствора для инъекции
Леупролелин, ацетат.
Прочитайте внимательно весь прошпект перед началом использования этого лекарственного средства, поскольку он содержит важную информацию для вас.
|
Содержание прошпекта:
- Что такое Элигард полугодовой и для чего он используется
- Что вам нужно знать перед началом использования Элигарда полугодового
- Как использовать Элигард полугодовой
- Возможные побочные эффекты
- Хранение Элигарда полугодового
- Содержание упаковки и дополнительная информация
1. Что такое Элигард полугодовой и для чего он используется
Активное вещество Элигарда полугодового относится к группе аналогов гонадотропин-рилизинг гормона. Эти лекарственные средства используются для снижения производства определенных половых гормонов (тестостерона).
Элигард полугодовой используется для лечения рака предстательной железыс метастазами, зависимого от гормонов, у взрослых мужчин и для лечения рака предстательной железы с высоким риском без метастазов, зависимого от гормонов, в комбинации с радиотерапией.
2. Что вам нужно знать перед началом использования Элигарда полугодового
Не используйте Элигард полугодовой
- Если вы женщина или ребенок.
- Если вы гиперчувствительны (аллергичны)к активному веществу ацетату леупролелина, к лекарственным средствам с подобной активностью к натуральному гонадотропин-рилизинг гормону или к любому из других компонентов Элигарда полугодового (перечисленных в разделе 6).
- После хирургического удаления яичек, поскольку в этом случае Элигард полугодовой не приводит к дальнейшему снижению концентрации тестостерона в крови.
- Как единственное лечение, если вы испытываете симптомы, связанные с давлением на спинной мозг или с опухолью в позвоночнике. В этом случае Элигард полугодовой можно использовать только в комбинации с другими лекарственными средствами для лечения рака предстательной железы.
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом, фармацевтом или медсестрой перед началом использования Элигарда полугодового
- Если у вас есть: любые сердечно-сосудистые расстройства, включая проблемы с сердечным ритмом (аритмию), или если вы проходите лечение лекарственными средствами для коррекции этих расстройств. Риск проблем с сердечным ритмом может увеличиться при использовании Элигарда полугодового.
- Если у вас есть затруднения при мочеиспускании. Вас необходимо будет внимательно наблюдать в течение первых недель лечения.
- Если вы начинаете испытывать давление на спинной мозг или затруднения при мочеиспускании. Поскольку были сообщения о тяжелых случаях (связанных с другими лекарственными средствами, имеющими подобный механизм действия, как Элигард полугодовой) давления на спинной мозг и сужения мочеточников, что может способствовать появлению симптомов, подобных параличу. Если возникают эти осложнения, необходимо начать стандартное лечение.
- Если вы испытываете, в течение двух недель после приема Элигарда полугодового, внезапную головную боль, рвоту, изменение психического состояния и иногда сердечную недостаточность, сообщите об этом вашему врачу или медицинской команде. Редко, эти случаи были сообщены с другими лекарственными средствами, имеющими подобный механизм действия, как Элигард полугодовой, и известны как апоплексия гипофиза.
- Если у вас есть сахарный диабет(высокие уровни глюкозы в крови). Вас необходимо будет регулярно контролировать во время лечения.
- Лечение Элигардом полугодовым может увеличить риск переломов из-за остеопороза (снижения плотности костей).
- Были сообщения о случаях депрессии у пациентов, принимавших Элигард полугодовой. Если вы принимаете Элигард полугодовой и начинаете испытывать депрессивное состояние, сообщите об этом вашему врачу.
- Были сообщения о случаях сердечно-сосудистых эффектов у пациентов, принимавших подобные лекарственные средства, как Элигард полугодовой, что не связано с этими лекарственными средствами. Если вы принимаете Элигард полугодовой и начинаете испытывать сердечно-сосудистые симптомы, сообщите об этом вашему врачу.
- Были сообщения о случаях эпилептических приступов у пациентов после введения Элигарда полугодового. Если вы принимаете Элигард полугодовой и начинаете испытывать эпилептические приступы, сообщите об этом вашему врачу.
- Свяжитесь с вашим врачом немедленно, если у вас есть сильные или повторяющиеся головные боли, проблемы со зрением или звоном в ушах.
- У вас есть жировая дегенерация печени.
Были сообщения о тяжелых кожных высыпаниях, включая синдром Стивенса-Джонсона/токсическую эпидермальную некролиз (СД/ТЭН), в связи с леупролелином. Прекратите использование леупролелина и немедленно обратитесь за медицинской помощью, если вы заметите какие-либо симптомы, связанные с этими тяжелыми кожными реакциями, описанные в разделе 4.
Осложнения в начале лечения
В течение первой недели лечения обычно наблюдается кратковременное увеличение мужского полового гормона, тестостерона, в крови. Это может привести к временному ухудшениюсимптомов, связанных с заболеванием, и также к появлению новых симптомов, которые не были испытаны до этого момента. Эти симптомы включают, в частности, боль в костях, расстройства мочеиспускания, давление на спинной мозг или наличие крови в моче. Эти симптомы обычно проходят при продолжении лечения. Если симптомы не проходят, необходимо обратиться к врачу.
Если вы не улучшаетесь при использовании Элигарда полугодового
Определенная часть пациентов будет иметь опухоли, которые не чувствительны к снижению концентрации тестостерона в крови. Если вы считаете, что эффект Элигарда полугодового не такой, как ожидается, сообщите об этом вашему врачу.
Использование Элигарда полугодового с другими лекарственными средствами
Это лекарственное средство может взаимодействовать с некоторыми лекарственными средствами, используемыми для лечения проблем с сердечным ритмом (например, хинидин, прокаинамид, амиодарон и соталол) или может увеличить риск проблем с сердечным ритмом при использовании с определенными лекарственными средствами (например, метадон, используемый для обезболивания и как часть программы детоксикации, моксифлоксацин, антипсихотические препараты, используемые для лечения тяжелых психических заболеваний).
Сообщите вашему врачу или фармацевту, если вы используете или недавно использовали любое другое лекарственное средство, включая те, которые можно купить без рецепта.
Беременность и лактация
Это лекарственное средство противопоказано женщинам.
Вождение и использование машин
Усталость, головокружение и расстройства зрения являются возможными побочными эффектами лечения Элигардом полугодовым или могут возникнуть из-за заболевания. Если вы испытываете эти побочные эффекты, будьте осторожны при вождении или использовании машин.
3. Как использовать Элигард полугодовой
Доза
Следуйте точно инструкциям по введению этого лекарственного средства, указанным вашим врачом или фармацевтом. В случае сомнений проконсультируйтесь с вашим врачом или фармацевтом.
Если ваш врач не назначил иное, это лекарственное средство вводится один разкаждые шесть месяцев.
Раствор для инъекции образует депо активного вещества, из которого происходит непрерывное высвобождение активного вещества, ацетата леупролелина, в течение шести месяцев.
Дополнительные исследования
Ответ на лечение этим лекарственным средством должен быть проверен вашим врачом путем проверки определенных клинических значений и определения концентрации в крови так называемого специфического антигена предстательной железы (ПСА).
Способ введения
Это лекарственное средство должно вводиться только вашим врачомили медсестрой. Они будут отвечать за его подготовку.
После подготовки Элигард полугодовой вводится в виде подкожной инъекции (инъекции в ткани под кожей). Внутриартериальная (в артерию) или внутривенная (в вену) инъекция должна быть полностью исключена. Как и в случае с другими активными веществами, вводимыми подкожно, место инъекции должно меняться периодически.
Если вы получите больше Элигарда полугодового, чем должно быть
Поскольку обычно инъекцию вводит ваш врач или квалифицированный персонал, не ожидается, что произойдет передозировка.
Если, тем не менее, была введена большая доза, чем предполагалось, свяжитесь с вашим врачом, чтобы он мог контролировать вас особенно и назначить дополнительное лечение, если это необходимо, или проконсультируйтесь с Центром токсикологической информации. Телефон 91 562 04 20.
Если вы пропустите введение Элигарда полугодового
Поговорите с вашим врачом, если вы считаете, что пропустили полугодовую инъекцию лекарственного средства.
Эффекты при прекращении лечения Элигардом полугодовым
Как правило, лечение рака предстательной железы этим лекарственным средством является длительным. Поэтому лечение не должно быть прервано, даже если симптомы улучшаются или полностью исчезают.
Если лечение Элигардом полугодовым будет прервано преждевременно, может произойти ухудшение симптомов, связанных с заболеванием.
Не прерывайте лечение преждевременно без предварительной консультации с вашим врачом.
Если у вас есть какие-либо другие вопросы об использовании этого лекарственного средства, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой.
4. Возможные побочные эффекты
Как и все лекарственные средства, Элигард полугодовой может вызывать побочные эффекты, хотя не все люди их испытывают.
Побочные эффекты, которые были обнаружены во время лечения Элигардом полугодовым, в основном связаны с специфическим эффектом активного вещества, ацетата леупролелина, а именно увеличением и снижением определенных гормонов. Побочные эффекты, которые описываются наиболее часто, являются приливами (приблизительно у 58% пациентов), тошнотой, общим недомоганием и усталостью, а также временной местной раздражением в месте инъекции.
Побочные эффекты в начале лечения
В течение первых недель лечения Элигардом полугодовым симптомы заболевания могут ухудшиться, поскольку в начале лечения происходит кратковременное увеличение мужского полового гормона, тестостерона, в крови. Поэтому ваш врач может назначить вам подходящий антиандроген (вещество, ингибирующее действие тестостерона) на начальном этапе лечения для снижения возможных нежелательных эффектов. (См. также раздел 2Прежде чем использовать Элигард полугодовой, Осложнения в начале лечения).
Местные побочные эффекты
Местные побочные эффекты, которые были описаны после инъекции Элигарда полугодового, обычно являются теми, которые часто ассоциируются с подобными препаратами, вводимыми подкожно (препаратами, вводимыми в ткань под кожей). Легкое жжение сразу после инъекции очень часто встречается. Зуд и боль после инъекции, а также синяки в месте инъекции часто встречаются. Покраснение кожи в месте инъекции часто сообщается. Загустение тканей и язвы редки.
Эти местные побочные эффекты после подкожной инъекции обычно легкие и имеют короткую продолжительность. Они не повторяются между отдельными инъекциями.
Очень частые побочные эффекты (могут возникать более чем у 1 из 10 человек)
- Приливы
- Спонтанные кровотечения на коже или слизистых оболочках, покраснение кожи
- Усталость, побочные эффекты, связанные с инъекцией (См. также местные побочные эффекты выше)
Частые побочные эффекты (могут возникать до 1 из 10 человек)
- Назофарингит (симптомы простуды)
- Тошнота, общее недомогание, диарея, воспаление желудка и кишечника (гастроэнтерит/колит)
- Зуд, ночная потливость
- Боль в суставах
- Нерегулярное мочеиспускание (также ночью), затруднения при начале мочеиспускания, боль при мочеиспускании, снижение объема мочи
- Чувствительность молочных желез, воспаление молочных желез, снижение размера яичек, боль в яичках, бесплодие, эректильная дисфункция, снижение размера полового члена
- Ригидность (эпизоды чрезмерного дрожания с высокой температурой), слабость
- Удлинение времени кровотечения, изменения в анализах крови, снижение количества красных кровяных телец/низкий уровень красных кровяных телец
Редкие побочные эффекты (могут возникать до 1 из 100 человек)
- Инфекция мочевыводящих путей, местная кожная инфекция
- Ухудшение сахарного диабета
- Аномальные сны, депрессия, снижение либидо
- Головокружение, головная боль, расстройства чувствительности кожи, бессонница, расстройства вкуса, расстройства обоняния
- Гипертония (повышение артериального давления), гипотония (снижение артериального давления)
- Затруднение дыхания
- Запор, сухость во рту, диспепсия (плохая пищеварение, симптомы переполнения желудка, боль в желудке, отрыжка, тошнота, рвота, ощущение жжения в желудке), рвота
- Чувство жара, увеличение потливости
- Боль в нижней части спины, мышечные спазмы
- Гематурия (наличие крови в моче)
- Спазмы мочевого пузыря, частое мочеиспускание, невозможность мочеиспускания
- Увеличение молочной железы у мужчин, импотенция
- Летаргия (сонливость), боль, температура
- Увеличение веса
- Потеря равновесия, головокружение
- Потеря мышечной массы/потеря мышечной ткани после длительного использования
Очень редкие побочные эффекты (могут возникать до 1 из 1 000 человек)
- Аномальные непроизвольные движения
- Внезапная потеря сознания, обморок
- Вздутие, отрыжка
- Потеря волос, кожная сыпь (прыщи на коже)
- Боль в груди
- Язвы в месте инъекции
Очень редкие побочные эффекты (могут возникать до 1 из 10 000 человек)
- Некроз в месте инъекции
Неизвестно (частота не может быть оценена из доступных данных)
- Изменения на электрокардиограмме (удлинение интервала QT)
- Воспаление легких, легочная болезнь
- Идиопатическая внутричерепная гипертония (повышение внутричерепного давления вокруг мозга, характеризующееся головными болями, двоением в глазах и другими зрительными симптомами, звоном в ушах)
- Если вы заметите на теле круглые или кольцевидные пятна красноватого цвета, часто с пузырьками в центре, отслоение кожи, язвы во рту, горле, носу, гениталиях и глазах. Эти тяжелые кожные высыпания могут быть предшествованы лихорадкой и симптомами, подобными гриппу (синдром Стивенса-Джонсона/токсическая эпидермальная некролиз)
- Покраснение кожи и сыпь с зудом (токсическая кожная эритема)
- Кожная реакция, вызывающая прыщи или красные пятна на коже, которые могут напоминать мишень, с красным центром, окруженным кольцами более светлого красного цвета (эритема многоформная)
Другие побочные эффекты
Другие побочные эффекты, опубликованные в связи с лечением леупролелином, активным веществом Элигарда полугодового, являются отеком (накоплением жидкости в ткани, проявляющимся как отек рук и ног), легочной эмболией (производящей симптомы, такие как чувство нехватки воздуха, затруднение дыхания и боль в груди), сердечными перебоями (восприятием сердечных сокращений), мышечной слабостью, ознобом, кожной сыпью и потерей памяти, а также ухудшением зрения. Можно ожидать увеличение потери костной ткани (остеопороза) после длительного лечения Элигардом полугодовым. Из-за остеопороза риск переломов увеличивается.
Редко были сообщены тяжелые аллергические реакции, вызывающие затруднение дыхания или чувство головокружения после введения продуктов той же классификации, что и Элигард полугодовой.
Были сообщены случаи эпилептических приступов после введения продуктов той же классификации, что и Элигард полугодовой.
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой, даже если это возможные побочные эффекты, которые не указаны в этом прошпекте. Вы также можете сообщить об этом напрямую через Испанскую систему фармаковигиланса для лекарственных средств для человека: www.notificaRAM.es. Сообщая о побочных эффектах, вы можете внести свой вклад в предоставление более полной информации о безопасности этого лекарственного средства.
5. Хранение Элигарда полугодового
Храните это лекарственное средство в недоступном для детей месте.
Не используйте это лекарственное средство после даты истечения срока годности, указанной на упаковке после CAD. Дата истечения срока годности - последний день месяца, указанного.
Инструкции по хранению
Храните в холодильнике (при температуре между 2°C и 8°C).
Храните в оригинальной упаковке для защиты от влаги.
Этот продукт должен быть при комнатной температуре перед инъекцией. Выньте из холодильника примерно за 30 минут до использования. Как только выньте из холодильника, этот продукт можно хранить в оригинальной упаковке при комнатной температуре (ниже 25°C) в течение 4 недель.
Как только упаковка открыта, продукт должен быть подготовлен немедленно и использован сразу. Только для одноразового использования.
Инструкции по утилизации неиспользованных или просроченных упаковок Элигарда полугодового
Лекарственные средства не должны выбрасываться в канализацию или мусор. Поместите упаковки и лекарственные средства, которые вам больше не нужны, в пункт SIGRE аптеки. Если у вас есть сомнения, спросите вашего фармацевта, как избавиться от упаковок и лекарственных средств, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержимое упаковки и дополнительная информация
Состав Элигарда семестр
Активное вещество - ацетат леупрорелина.
Предварительно заполненный шприц (шприц Б) содержит 45 мг ацетата леупрорелина.
Другие компоненты - полимолочная кислота (85:15) и N-метил-2-пирролидон в предварительно заполненном шприце с раствором для инъекции (шприц А).
Внешний вид Элигарда семестр и содержимое упаковки
Порошок и раствор для инъекции.
Доступно в следующих упаковках:
- Упаковка типа термоформованная лоток и стерильная игла калибра 18, вставленная в картонную подставку. Лоток содержит пакет с десикантом и предварительно подключенную систему шприца, состоящую из:
- шприца А, предварительно заполненного растворителем
- шприца Б, предварительно заполненного порошком и соединителем с кнопкой для шприца А и Б
- Множественная упаковка, содержащая 2 комплекта предварительно подключенной системы шприца.
Возможно, не все размеры упаковок будут продаваться.
Владелец разрешения на маркетинг
Recordati Industria Chimica e Farmaceutica S.p.A.
Виа Маттео Чивитали, 1
20148 Милан
Италия
Производитель
Recordati Industria Chimica e Farmaceutica S.p.A.
Виа Маттео Чивитали, 1
20148 Милан
Италия
Для получения дополнительной информации о этом лекарственном средстве можно обратиться к местному представителю владельца разрешения на маркетинг:
Casen Recordati, S.L.
Автовия де Логроньо, км 13,300
50180 Утебо - Сарагоса
Испания
Это лекарственное средство разрешено в государствах-членах Европейского экономического пространства под следующими названиями:
Австрия: Eligard Depot 45 мг
Бельгия: Depo-Eligard 45 мг
Болгария: Eligard 45 мг
Кипр: Eligard
Чехия: Eligard
Дания: Eligard
Эстония: Eligard
Финляндия: Eligard
Франция: Eligard 45 мг
Германия: Eligard 45 мг
Венгрия: Eligard 45 мг
Исландия: Eligard
Ирландия: Eligard 45 мг
Италия: Eligard
Латвия: Eligard 45 мг
Литва: Eligard 45 мг
Люксембург: Depo-Eligard 45 мг
Нидерланды: Eligard 45 мг
Норвегия: Eligard
Польша: Eligard 45 мг
Португалия: Eligard 45 мг
Румыния: Eligard 45 мг
Словакия: Eligard 45 мг
Словения: Eligard 45 мг
Испания: Eligard семестр 45 мг
Швеция: Eligard
Дата последнего пересмотра этой инструкции: 10/2024
Подробная и актуальная информация о этом лекарственном средстве доступна на сайте Агентства по лекарственным средствам и медицинским изделиям Испании (AEMPS) http://www.aemps.es/
Эта информация предназначена только для медицинских специалистов:
Подождите, пока лекарство достигнет комнатной температуры, вынув его из холодильника примерно за 30 минут до использования.
Сначала подготовьте пациента к инъекции, затем подготовьте лекарство, следуя инструкциям, указанным ниже. Если лекарство не подготовлено с помощью правильной техники, его не следует вводить, поскольку это может привести к отсутствию клинического эффекта из-за неправильного восстановления.
Шаг 1
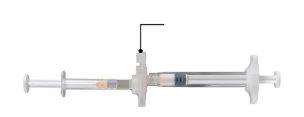
В чистой зоне откройте лоток, сняв алюминий с угла, чтобы извлечь содержимое. Утилизируйте пакет с десикантом. Удалите предварительно подключенную систему шприца (Рисунок 1.1) из лотка. Откройте упаковку безопасности иглы (Рисунок 1.2), сняв бумажную вкладку. Примечание:шприц А и шприц Б еще не должны быть выровнены.
Рисунок 1.1 Содержимое лотка: предварительно подключенная система шприца | Рисунок 1.2 Под лотком: игла безопасности и колпачок |
|
|
Шаг 2
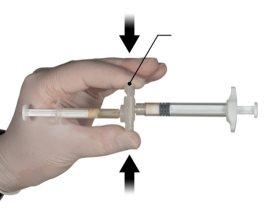
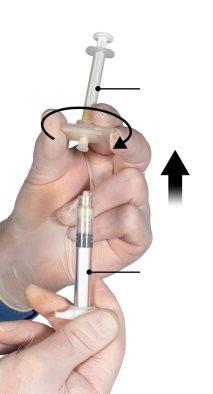
Держите кнопку соединителя с помощью указательного и большого пальцев и нажмите (Рисунок 2) до тех пор, пока не услышите щелчок. Два шприца будут выровнены. Не требуется никакого специального положения системы шприцев для активации соединителя. Не сгибайте систему шприцев (пожалуйста, обратите внимание, что часть лекарства может быть потеряна, если шприцы будут частично отсоединены).
Рисунок 2 |
|
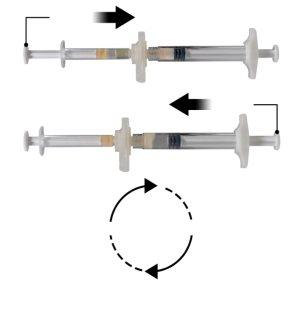
Шаг 3
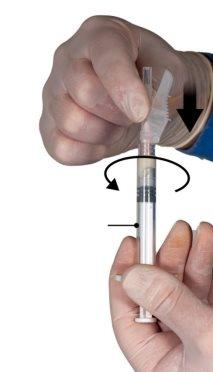
Держите шприцы в горизонтальном положении, перелейте жидкое содержимое шприца А в порошок ацетата леупрорелина, содержащийся в шприце Б. Тщательно перемешайте продукт в течение 60 циклов, мягко нажимая содержимое обоих шприцев вперед и назад между шприцами (один цикл - это толчок поршня для шприца А и толчок поршня для шприца Б) в горизонтальном положении, чтобы получить однородную и вязкую раствор (Рисунок 3). Не сгибайте систему шприцев (пожалуйста, обратите внимание, что часть лекарства может быть потеряна, если шприцы будут частично отсоединены).
Рисунок 3 |
|
Когда раствор тщательно перемешан, вязкая раствор будет иметь цвет от бесцветного до белого-желтого (что может включать оттенки от белого до желтого).
Важно: после перемешивания немедленно перейдите к следующему шагу, поскольку вязкость продукта увеличивается со временем. Не охлаждайте лекарство после восстановления.
Пожалуйста, обратите внимание: лекарство должно быть перемешано, как описано; встряхивание не даст подходящей смеси.
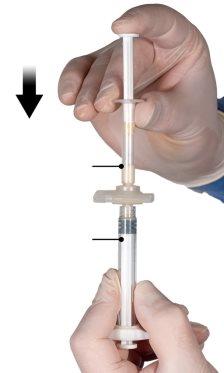
Шаг 4
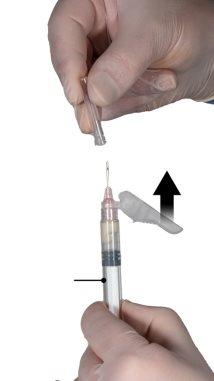
После перемешивания держите шприцы вертикально с шприцем Б внизу. Шприцы должны оставаться хорошо соединенными. Перелейте все содержимое в шприц Б (широкий шприц), нажимая поршень шприца А и слегка оттягивая поршень шприца Б (Рисунок 4).
Рисунок 4 |
|
Шаг 5
Держите соединитель и отсоедините его от шприца Б, пока поршень шприца А полностью нажат вниз. Убедитесь, что содержимое не вытечет, поскольку в противном случае игла не будет правильно установлена, когда будет соединена.
Пожалуйста, обратите внимание: при подготовке может остаться большая воздушная пузырь или несколько небольших - это допустимо. Пожалуйста, не удаляйте воздушные пузырьки из шприца Б в этот момент, поскольку это приведет к потере лекарства!
Рисунок 5 |
|
Шаг 6
- Держите шприц Б в вертикальном положении и держите белый поршень назад, чтобы избежать потери лекарства.
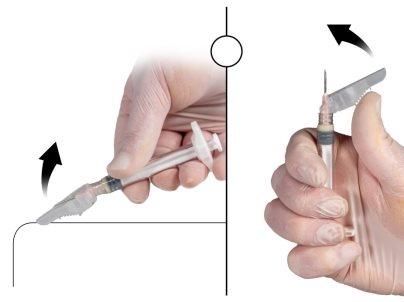
- Зафиксируйте иглу безопасности на шприце Б, держа шприц и мягко поворачивая иглу по часовой стрелке, примерно на три четверти поворота, пока игла не будет зафиксирована (Рисунок 6).
Не перетягивайте, поскольку это может привести к растрескиванию конуса иглы и потере лекарства во время инъекции. Колпачок безопасности также может быть поврежден, если игла будет слишком сильно закручена.
Не используйте лекарство, если конус иглы треснул, поврежден или содержимое вытекло. Поврежденную иглу не следует заменять или ремонтировать, и лекарство не должно быть введено. Все элементы устройства для введения должны быть утилизированы безопасно.
Если конус иглы поврежден, замените лекарство на новое.
Рисунок 6 |
|
Шаг 7
Немедленно перед введением отодвиньте защитный колпачок от иглы и удалите защитный колпачок иглы (Рисунок 7).
Важно: не манипулируйте механизмом иглы безопасности до введения. Если игла, кажется, повреждена или имеет утечки, продукт не должен быть использован. Поврежденную иглу не следует заменять, и лекарство не должно быть введено. В случае повреждения иглы используйте другой комплект Элигарда.
Рисунок 7 |
|
Шаг 8
Перед введением удалите любую большуювоздушную пузырь из шприца Б. Введите лекарство подкожно, держа защитный колпачок подальше от иглы.
Процедура введения:
- Выберите место инъекции на животе, верхней части ягодиц или в другом месте с достаточным количеством подкожной ткани, не имеющей избыточного пигмента, узлов, поражений или волос и не использованной недавно.
- Очистите область места инъекции с помощью салфетки с алкоголем (не включена).
- Используя большой и указательный пальцы, зажмите и соберите кожу вокруг места инъекции.
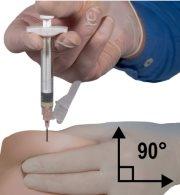
- Используя свою доминирующую руку, быстро вставьте иглу под углом 90° относительно поверхности кожи. Глубина проникновения будет зависеть от количества и ширины подкожной ткани и длины иглы. После введения иглы отпустите кожу.
- Введение лекарства, медленно и равномерно нажимая на поршень, пока шприц не будет пустым. Убедитесь, что введена вся доза лекарства из шприца Б, прежде чем удалить иглу.
- Удалите иглу быстро под тем же углом 90°, который был использован для введения, сохраняя давление на поршень.
Рисунок 8

Шаг 9
После инъекции блокируйте механизм безопасности, используя любой из методов активации, упомянутых ниже.
- Закрытие на плоской поверхности
Нажмите защитный колпачок с помощью скользящего механизма вниз, на плоской поверхности (Рисунок 9а), чтобы закрыть иглу и заблокировать колпачок.
Проверьте положение блокировки с помощью слышимого и ощутимого "щелчка". Положение блокировки полностью закроет кончик иглы.
- Закрытие с помощью большого пальца
Поместив большой палец на защитный колпачок (Рисунок 9б), закройте кончик иглы и заблокируйте колпачок.
Проверьте положение блокировки с помощью слышимого и ощутимого "щелчка". Положение блокировки полностью закроет кончик иглы.
Рисунок 9а Закрытие на плоской поверхности | Рисунок 9б Закрытие с помощью большого пальца |
|
После блокировки защитного колпачка немедленно утилизируйте иглу и шприц в уполномоченном контейнере для острых предметов.

Сколько стоит ЭЛИГАРД СЕМЕСТРАЛЬ 45 мг ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА в Испании в 2026 году?
Средняя цена на ЭЛИГАРД СЕМЕСТРАЛЬ 45 мг ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА в январь, 2026 года составляет около 549.08 евро. Финальная стоимость может зависеть от региона, конкретной аптеки и рецептурного статуса. Для точной информации лучше проверить онлайн или в ближайшей аптеке.
- Страна регистрации
- Средняя цена в аптеках549.08 EUR
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги ЭЛИГАРД СЕМЕСТРАЛЬ 45 мг ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРАФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 42 мгАктивное вещество: leuprorelinПроизводитель: Accord Healthcare S.L.U.Требуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 22,5 мгАктивное вещество: leuprorelinПроизводитель: Recordati Industria Chimica E Farmaceutica S.P.A.Требуется рецептФорма выпуска: ИМПЛАНТ, 5 мгАктивное вещество: leuprorelinПроизводитель: Sandoz Farmaceutica S.A.Требуется рецепт
Аналоги ЭЛИГАРД СЕМЕСТРАЛЬ 45 мг ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог ЭЛИГАРД СЕМЕСТРАЛЬ 45 мг ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА в Польша
Аналог ЭЛИГАРД СЕМЕСТРАЛЬ 45 мг ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА в Украина
Врачи онлайн по ЭЛИГАРД СЕМЕСТРАЛЬ 45 мг ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на ЭЛИГАРД СЕМЕСТРАЛЬ 45 мг ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА – по решению врача и с учетом местных правил.