
EBASTINE SANDOZ 10 mg ORALLY DISINTEGRATING TABLETS

How to use EBASTINE SANDOZ 10 mg ORALLY DISINTEGRATING TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Ebastina Sandoz 10 mg Oral Dispersible Tablets EFG
Ebastina Sandoz 20 mg Oral Dispersible Tablets EFG
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Ebastina Sandoz and what is it used for
- What you need to know before you take Ebastina Sandoz
- How to take Ebastina Sandoz
- Possible side effects
- Storage of Ebastina Sandoz
- Contents of the pack and other information
1. What is Ebastina Sandoz and what is it used for
Ebastine is an antihistamine that helps to relieve the symptoms of allergy such as: sneezing, runny nose, tearful eyes, itchy skin rashes.
Ebastine is used in adults and children over 12 years of age to relieve the symptoms of seasonal allergic rhinitis (hay fever) and perennial allergic rhinitis, including cases with allergic conjunctivitis.
Ebastine 10 mg tablets are also used in adults over 18 years of age to relieve the symptoms of itching and hives in cases of urticaria (hives).
2. What you need to know before you take Ebastina Sandoz
Do not take Ebastina Sandoz:
- If you are allergic to ebastine or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before taking Ebastina Sandoz if:
- You are already taking certain antibiotics or medicines used to treat fungal infections: see section “Taking Ebastina Sandoz with other medicines” below,
- You have severe liver function impairment (liver failure).
Children and adolescents
This medicine should only be used in children from 12 years of age. Do not give this medicine to children under 12 years because the safety and efficacy in this age group have not been established.
Taking Ebastina Sandoz with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
If you take ebastine with erythromycin (an antibiotic), ketoconazole or itraconazole (active substances for the treatment of fungal infections), it may increase the levels of ebastine in the blood.
Taking ebastine and rifampicin (an antitubercular agent) together may lead to a decrease in ebastine blood levels and therefore a decrease in its effect.
It is not recommended to use ebastine at the same time as clarithromycin or josamycin (antibiotics).
Taking Ebastina Sandoz with food and drinks
Ebastina Sandoz can be taken with or without food.
Pregnancy, breast-feeding and fertility
There are no sufficient data on the safety for the fetus of the use of this medicine in humans. For this reason, ebastine should only be taken during pregnancy if your doctor considers that the benefit outweighs the possible risks.
Do not take ebastine if you are breast-feeding, as it is not known whether the active substance is excreted in breast milk.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Most patients taking ebastine can drive or perform other activities that require attention. However, as with other medicines, you should check your individual reaction after taking ebastine before driving or performing complex activities, as some patients may experience drowsiness or dizziness.
Ebastina Sandoz contains lactose, aspartame, and sodium.
This medicine contains lactose. If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
This medicine contains 2.5 mg of aspartame (E951) per 10 mg tablet and 5 mg of aspartame (E951) per 20 mg tablet. Aspartame is a source of phenylalanine, which may be harmful for people with phenylketonuria (a rare genetic disorder in which phenylalanine accumulates in the body because it is not properly broken down).
This medicine contains less than 23 mg of sodium (1 mmol) per oral dispersible tablet, which is essentially “sodium-free”.
3. How to take Ebastina Sandoz
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
The recommended dose is:
Indication | Age | Dose |
Allergic rhinitis In case of intense symptoms | Children from 12 years of age and adults | One ebastine 10 mg tablet (10 mg of ebastine) once a day. Two ebastine 10 mg tablets or one ebastine 20 mg tablet (20 mg of ebastine) once a day. |
Urticaria | Adults over 18 years of age | One ebastine 10 mg tablet (10 mg of ebastine) once a day. |
In patients with impaired renal function, no dose adjustment is necessary.
In patients with mild or moderate liver function impairment, no dose adjustment is necessary.
There is no experience with doses greater than 10 mg in patients with severe liver failure, therefore the dose should not exceed 10 mg in these patients.
Do not push the tablet out of the blister, as it may break.
Each blister contains the tablets separately in blisters separated by a perforation line.
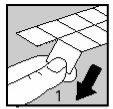
Cut one of the blisters along the dotted line (Figure 1)
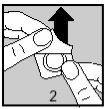
Then, carefully remove the aluminum foil, separating it at the corner indicated by an arrow (Figures 2 and 3).
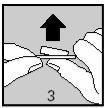
Keep your hands dry to remove the tablet from the blister.
Place your tablet on your tongue where it dissolves in a matter of seconds, no water or other liquid is needed.
Ebastina can be taken with or without food.
Your doctor will indicate the duration of the treatment.
If you take more Ebastina Sandoz than you should
There is no specific antidote for the active substance ebastine.
In case of overdose with ebastine, please consult your doctor. Depending on the severity of the poisoning, your doctor will initiate the necessary measures (monitoring of vital functions, including ECG monitoring for at least 24 hours, symptomatic treatment, and gastric lavage) if necessary.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to take Ebastina Sandoz
Do not take a double dose to make up for forgotten doses.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop taking Ebastina Sandoz and contact your doctor immediately or go to the nearest hospital if you experience:
Severe allergic reaction that causes itching and swelling of the face, tongue, or throat and may cause difficulty swallowing or breathing.
Severe allergic reactions with ebastine are rare (may affect up to 1 in 1,000 people).
Other side effects include:
Very common side effectsmay affect more than 1 in 10 people
- headache.
Common side effectsmay affect up to 1 in 10 people
- drowsiness
- dry mouth.
Rare side effectsmay affect up to 1 in 1,000 people
- nervousness, insomnia,
- dizziness, decreased sense of touch, altered taste,
- palpitations, rapid heartbeat,
- abdominal pain, vomiting, nausea, indigestion,
- liver inflammation (hepatitis), bile elimination problems (cholestasis), abnormal liver function tests,
- skin rash, hives, skin inflammation,
- menstrual disorders,
- edema (swelling due to fluid accumulation in tissues), weakness (asthenia).
Frequency not known: cannot be estimated from the available data
- weight gain,
- increased appetite.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Surveillance System for Human Use: http://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ebastina Sandoz
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the blister and carton after EXP. The expiry date is the last day of the month stated.
Store in the original package to protect from light.
Medicines should not be disposed of via wastewater or household waste. Return any unused medicine to a pharmacy for proper disposal. This will help protect the environment.
6. Contents of the pack and other information
Composition of Ebastina Sandoz
- The active substance is ebastine.
- Ebastina Sandoz 10 mg: each oral dispersible tablet contains 10 mg of ebastine.
- Ebastina Sandoz 20 mg: each oral dispersible tablet contains 20 mg of ebastine.
- The other ingredients are microcrystalline cellulose, lactose monohydrate, corn starch, sodium croscarmellose, aspartame (E951), peppermint flavor, colloidal anhydrous silica, and magnesium stearate.
Appearance and packaging
Oral dispersible tablet.
Ebastina Sandoz 10 mg:
White, biconvex, round tablets, approximately 6.7 mm in diameter, marked with “E10” on one face and smooth on the other face.
Ebastina Sandoz 20 mg:
White, biconvex, round tablets, approximately 9.2 mm in diameter, marked with “E20” on one face and smooth on the other face.
The tablets are packaged in OPA/Alu/PVC/Alu blisters packaged in cardboard boxes.
Package sizes:
Ebastina Sandoz 10 mg:
10, 20, 30, 40, 50, 90, 98, or 100 oral dispersible tablets.
Ebastina Sandoz 20 mg:
10, 15, 20, 30, 40, 50, 98, or 100 oral dispersible tablets.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Manufacturing authorization holder
Sandoz Farmacéutica, S.A.
Centro Empresarial Parque Norte
Edificio Roble
C/ Serrano Galvache, 56
28033 Madrid
Spain
Manufacturer
Lek Pharmaceuticals d.d.
Verovškova 57,
1526 Ljubljana
Slovenia
TEVA Pharmaceutical Works Private Limited Company
Pallagi út 13, 4042 Debrecen
Hungary
or
Teva Pharma, S.L.U.
C/Anabel Segura 11, Edificio Albatros B, 1st floor, Alcobendas, 28108
Madrid
Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
Belgium: Ebastine Sandoz 10 mg orodispergeerbare tabletten
Italy: Ebastina Sandoz
Sweden: Ebastine Sandoz, 10 mg munsönderfallande tablett
Ebastine Sandoz, 20 mg munsönderfallande tablett
Date of last revision of this leaflet:August 2020
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price4.4 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EBASTINE SANDOZ 10 mg ORALLY DISINTEGRATING TABLETSDosage form: TABLET, 10 mg ebastineActive substance: ebastineManufacturer: Laboratorios Almirall S.L.Prescription not requiredDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 10 mgActive substance: ebastineManufacturer: Laboratorios Almirall S.L.Prescription not requiredDosage form: TABLET, 20 mg ebastineActive substance: ebastineManufacturer: Laboratorios Almirall S.L.Prescription not required
Online doctors for EBASTINE SANDOZ 10 mg ORALLY DISINTEGRATING TABLETS
Discuss questions about EBASTINE SANDOZ 10 mg ORALLY DISINTEGRATING TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















