
CLUVOT 250 IU, POWDER AND SOLVENT FOR INJECTABLE SOLUTION OR INFUSION

How to use CLUVOT 250 IU, POWDER AND SOLVENT FOR INJECTABLE SOLUTION OR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Cluvot 250 UI
Powder and solvent for solution for injection or infusion.
Concentrate of human plasma-derived coagulation factor XIII
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
|
Contents of the package leaflet
- What is Cluvot and what is it used for
- What you need to know before you use Cluvot
- How to use Cluvot
- Possible side effects
- Storage of Cluvot
- Contents of the pack and other information
1. What is Cluvot and what is it used for
What is Cluvot
Cluvot is presented as a white powder and a solvent. The resulting solution should be administered by injection into a vein.
Cluvot is a product composed of human plasma-derived coagulation factor XIII (the liquid part of the blood), and has important functions in haemostasis (stopping bleeding).
What is Cluvot used for
Cluvot is used in adult and paediatric patients
- for the preventive treatment of hereditary factor XIII deficiency and
- for the perioperative treatment of surgical bleeding with congenital FXIII deficiency
.
2. What you need to know before you use Cluvot
The following sections contain information that your doctor should consider before administering Cluvot to you.
Do not use Cluvot:
- if you are allergic to the active substance or to any of the other ingredients of this medicine (listed in section 6).
Tell your doctor if you are allergic to any medicine or food.
Warnings and precautions
Consult your doctor, pharmacist or nurse before starting treatment with Cluvot.
- If you have experienced allergic reactions to FXIII coagulation factor in the past. You should take antihistamines and corticosteroids for prophylactic purposes if your doctor advises you to do so.
- When allergic or anaphylactic reactions (a severe allergic reaction that causes severe breathing difficulties or dizziness) occur, administration of Cluvot should be discontinued immediately (e.g., discontinue infusion). In case of shock, standard medical guidelines for its treatment should be followed.
- If you have recently experienced a thrombosis (blood clot), caution should be exercised due to the fibrin-stabilizing effect of FXIII.
- The formation of inhibitors (neutralizing antibodies) is a known complication of treatment and means that the treatment has stopped working. If your bleeding cannot be controlled with Cluvot, inform your doctor immediately. You should be carefully monitored for the development of any inhibitors.
Your doctor will carefully consider the benefit of treatment with Cluvot compared to the risk of these complications.
Viral safety
When medicines are prepared from human blood or plasma, certain measures are taken to prevent the transmission of infections to patients. These measures include:
- careful selection of blood and plasma donors to ensure that potential carriers of infections are excluded;
- testing of each donation and plasma pool for signs of viruses/infections;
- inclusion of measures during the processing of the blood or plasma to inactivate or remove viruses.
Despite these measures, when medicines prepared from human blood or plasma are administered, the possibility of transmitting infections cannot be totally excluded. This also applies to any unknown or emerging viruses or other types of infections.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus and hepatitis C virus, and for the non-enveloped hepatitis A virus and parvovirus B19.
It is strongly recommended that, each time you are administered Cluvot, your doctor records the name and batch number of the medicine (which is found on the carton).
Your doctor may recommend that you consider getting vaccinated against hepatitis A and B if you receive human plasma-derived products regularly or repeatedly.
Using Cluvot with other medicines
- Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
- So far, no interactions have been reported between human plasma-derived FXIII coagulation factor concentrate and other medicines.
- Cluvot should not be mixed with other medicines, diluents or solvents, except those indicated in section 6, and should be administered through a separate infusion line.
Pregnancy, breastfeeding and fertility
- If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before you are given this medicine.
- Limited data on the clinical use of Cluvot in pregnancy did not show adverse effects on pregnancy and perinatal or postnatal development. Therefore, if necessary, the use of Cluvot during pregnancy may be considered.
- No data are available on the excretion of Cluvot in human milk. However, based on its large molecular size, excretion into milk is unlikely, and due to its protein nature, absorption of intact molecules by the infant is also unlikely. Therefore, Cluvot can be used during breastfeeding.
- No fertility data are available.
Driving and using machines
No studies on the effects on the ability to drive and use machines have been performed.
Cluvot contains sodium
Be aware that Cluvot contains sodium. This is important if you are on a controlled sodium diet. Cluvot contains 124.4 to 195.4 mg (5.41 to 8.50 mmol) of sodium per dose (40 UI/body weight: for an average of 70 kg), if the recommended dose is applied (2,800 UI = 44.8 ml).
3. How to use Cluvot
- Cluvot will normally be administered to you by your doctor.
- Cluvot is intended for intravenous injection only.
Dosage
Your doctor will calculate the correct dose and decide how often you will be given Cluvot, taking into account how well you are responding to the treatment.
For more information, see the section "This information is intended only for healthcare professionals".
Overdose
No cases of overdose have been reported and are not expected, as the medicine is administered by healthcare professionals.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been observed rarely(affects more than 1 user in 10,000 and less than 1 user in 1,000):
- Allergic reactions, such as generalised urticaria (itchy swellings on the skin), skin rash, decrease in blood pressure (which can cause dizziness or fainting) and difficulty breathing.
- Increased temperature
The following side effects have been observed very rarely(affects less than 1 user in 10,000):
- Development of FXIII inhibitors.
If allergic reactionsoccur, administration of Cluvot should be discontinued immediately and appropriate treatment initiated. For the treatment of shock, standard medical guidelines should be followed.
Side effects in children and adolescents
It is expected that the side effects in children will be the same as in adults.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Cluvot
- Store in a refrigerator (between +2°C and +8°C).
- Do not freeze.
- Keep the vial in the outer packaging to protect it from light.
- Cluvot does not contain preservatives. The product should be used immediately after reconstitution. If not used immediately, it should not be stored for more than 4 hours at room temperature. Do not refrigerate or freeze the reconstituted solution.
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the label and carton. The expiry date is the last day of the month stated.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Cluvot Composition
The active ingredient is:
Concentrate of factor XIII of coagulation derived from human plasma that contains 250 UI per vial.
The other components are:
Human albumin, glucose monohydrate, sodium chloride, sodium hydroxide (in small quantities for pH adjustment)
Solvent:Water for injectable preparations
Appearance of the Product and Container Contents
Cluvot is presented as a white powder and is supplied with a solvent (water for injectable preparations).
The obtained solution must be colorless, transparent, or slightly opalescent. When exposed to light, it must not be turbid or contain residues (deposits/particles).
Presentation
A container of 250 UI that contains:
- 1 vial with powder
- 1 vial with 4 ml of water for injectable preparations
- 1 transfer device with 20/20 filter (Mix2Vial)
- Administration equipment (inner box):
- 1 disposable 5 ml syringe
- 1 venipuncture device
- 2 alcohol-impregnated swabs
- 1 non-sterile dressing
Marketing Authorization Holder and Manufacturer
CSL Behring GmbH
Emil-von-Behring-Strasse 76
35041 Marburg (Germany)
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
CSL Behring, S.A.
c/ Tarragona 157, 18th floor
08014 Barcelona (Spain)
This medication is authorized in the member states of the European Economic Area under the following names:Cluvot
Date of the last revision of this prospectus: October 2018.
Detailed and updated information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
_____________________________________________________________________________________
This information is intended only for healthcare professionals:
Posology and Method of Administration
Posology
1 ml is approximately equivalent to 62.5 UI, and 100 UI are equivalent to 1.6 ml, respectively.
Important:
The amount to be administered and the frequency of administration should always be guided by clinical efficacy in each particular case.
Dosage
The dosage regimen should be personalized according to the patient's body weight, analytical values, and clinical condition.
Usual prophylactic dosage regimen
Initial dose
- 40 international units (UI) per kg of body weight
- The injection rate must not exceed 4 ml per minute.
Subsequent dosage
- The dosage should be guided by the most recent minimum FXIII activity level, with administration every 28 days (4 weeks) to maintain a minimum FXIII activity level between 5 and 20%, approximately.
- The recommended dose adjustments of ± 5 UI per kg should be based on the minimum FXIII activity levels as shown in Table 1 and the patient's clinical condition.
- Dose adjustments should be made based on a specific and sensitive analysis to determine FXIII levels. Table 1 below describes an example of dose adjustment using the Berichrom® standard activity test.
Table 1: Dose adjustment using the Berichrom® activity test
Minimum FXIII activity level (%) | Dose change |
A minimum level of <5% | Increase by 5 units per kg |
Minimum level between 5% and 20% | No change |
Two minimum levels of > 20% | Decrease by 5 units per kg |
A minimum level of > 25% | Decrease by 5 units per kg |
The potency expressed in units is determined by the Berichrom® activity test, referred to the current international standard for blood coagulation factor XIII in plasma.
Therefore, one unit in this document is equivalent to one international unit.
Pre-surgical prophylaxis
After the patient's last usual prophylactic dose, if surgery is scheduled:
- Between 21 and 28 days later: administer the patient's total prophylactic dose immediately before surgery, and the next prophylactic dose should be administered 28 days later.
- Between 8 and 21 days later: an additional dose (total or partial) may be administered before surgery. The dose should be guided by FXIII activity levels and the patient's clinical condition and should be adjusted according to Cluvot's half-life.
- Within 7 days after the last dose: an additional dose may not be necessary.
Dose adjustments may differ from these recommendations and should be personalized based on FXIII activity levels and the patient's clinical condition. All patients should be closely monitored during and after surgery.
Therefore, it is recommended to monitor the increase in FXIII activity with an FXIII test. In the case of major surgery and severe bleeding, the goal is to achieve almost normal values (healthy individuals: 70% - 140%).
Pediatric Population
The posology and method of administration in children and adolescents are based on body weight and, therefore, are generally based on the same guidelines as for adults. The dose and frequency of administration for each person should always be guided by clinical efficacy and FXIII activity levels.
Elderly Population
The posology and method of administration in elderly individuals (> 65 years) have not been documented in clinical studies yet.
Method of Administration
General Instructions
- The solution must be transparent or slightly opalescent. After extracting and filtering the reconstituted product (see below), it should be visually inspected for the presence of foreign particles and discoloration before administration.
- Visibly turbid solutions or solutions that still contain flakes or particles should not be used.
- Reconstitution and extraction should be carried out under aseptic conditions.
Reconstitution
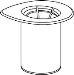
Bring the solvent to room temperature. Ensure that the caps have been removed from the product and solvent vials and that the stoppers have been treated with an aseptic solution and allowed to dry before opening the Mix2Vial container.
|
|
|
|
|
|
|
|
|
Discard the solvent vial with the blue Mix2Vial adapter attached. |
|
|
|
|
Transfer and Administration
|
by slowly pulling the plunger backward. |
|
|
Care should be taken to ensure that blood does not enter the syringe filled with the product, as there is a risk that the blood will coagulate in the syringe and therefore, fibrin clots will be administered to the patient.
The reconstituted solution must be administered intravenouslyin an independent injection/infusion line (provided with the product) through slow injection, at a rate that does not exceed 4 ml per minute.
The disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CLUVOT 250 IU, POWDER AND SOLVENT FOR INJECTABLE SOLUTION OR INFUSIONDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1500 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1000 IU - after reconstitution in 2 ml of water for injections, the dose is 500 IU/mlActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription required
Online doctors for CLUVOT 250 IU, POWDER AND SOLVENT FOR INJECTABLE SOLUTION OR INFUSION
Discuss questions about CLUVOT 250 IU, POWDER AND SOLVENT FOR INJECTABLE SOLUTION OR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions





 1
1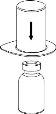 2
2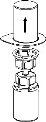 3
3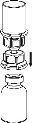 4
4 5
5 6
6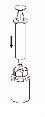 7
7 8
8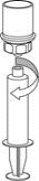 9
9









