
BILINA 0.5 mg/ml EYE DROPS IN SUSPENSION

How to use BILINA 0.5 mg/ml EYE DROPS IN SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
Bilina 0.5 mg/ml eye drops in suspension
Levocabastina
Read this leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any doubts, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Bilina and what is it used for
- What you need to know before using Bilina
- How to use Bilina
- Possible side effects
- Storage of Bilina
- Package contents and additional information
1. What is Bilina and what is it used for
Bilina 0.5 mg/ml eye drops in suspension contain levocabastina, which is an antihistamine used for the symptomatic treatment of allergic conjunctivitis in adults and in children and adolescents from 4 to less than 18 years of age.
Levocabastina is a highly selective antagonist of histamine H1 receptors. After application to the eyes, it provides rapid and long-lasting relief from the symptoms of allergic conjunctivitis (itching, redness, eyelid inflammation, tearing).
2. What you need to know before using Bilina
Do not use Bilina
- If you are allergic (hypersensitive) to levocabastina or any of the other components of this medication (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Bilina.
Children and adolescents
- The safety and efficacy of this medication have not been established in children under 4 years of age.
- Bilina should only be used to treat children and adolescents from 4 to less than 18 years of age.
Using Bilina with other medications
No interactions have been described.
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medication.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you might be pregnant, or plan to become pregnant, do not use Bilina, as safety studies have not been conducted in pregnant women, and Bilina passes into breast milk. Consult your doctor or pharmacist before using this medication.
Driving and using machines
Bilina does not cause sedation or interfere with psychomotor activity. Adverse reactions with Bilina eye drops, such as eye irritation, pain, swelling, itching, redness, burning sensation, tearing, or blurred vision, have been reported, which may affect vision. Therefore, caution is recommended when driving or operating machinery after applying Bilina eye drops.
Bilina eye drops contain benzalkonium chloride
This medication contains 15 mg of benzalkonium chloride per ml.
Benzalkonium chloride can be absorbed by soft contact lenses, altering their color. Remove contact lenses before using this medication and wait 15 minutes before putting them back in.
Benzalkonium chloride can cause eye irritation, especially if you have dry eye or other corneal diseases (the transparent layer on the front of the eye). Consult your doctor if you feel any unusual sensation, itching, or pain in the eye after using this medication.
This medication contains 14.04 mg of phosphates per ml.
If you have severe corneal damage (the transparent layer on the front of the eye), treatment with phosphates, in very rare cases, may cause blurred vision due to calcium accumulation.
3. How to use Bilina
- Dosage
Follow your doctor's instructions for administering this medication exactly. If you have any doubts, consult your doctor or pharmacist again.
Adults and children and adolescents from 4 to less than 18 years of age
The recommended dose is 1 drop of Bilina per eye, 2 times a day. The dose can be increased to 1 drop in each eye, 3 or 4 times a day. Treatment should continue until symptoms disappear.
Use in children under 4 years of age
The safety and efficacy of this medication have not been established. No data are available.
Elderly
No data are available on the use of levocabastina in the elderly.
- Instructions for use
Before the first use of Bilina, remove the seal from the container and note the opening date on the box.
Follow these steps:
- Shake the container well before removing the cap
- Tilt your head back as far as possible
- Press the bottle and carefully pour 1 drop into the inner corner of the eye. Pull the lower eyelid down while keeping your head tilted back, so the drops can reach the area between the eye and the eyelid. Try to blink to spread the drops across the entire eye.
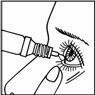 Repeat step 3 for the other eye.
Repeat step 3 for the other eye.
Do not touch the bottle to the eye to avoid introducing impurities into the remaining liquid. Do not use the drops for more than 1 month after opening the bottle for the first time.
Treatment should continue until symptoms improve.
If you use more Bilina than you should
If you accidentally drink the contents of a container, you may feel drowsy. In this case, it is recommended to drink plenty of non-alcoholic liquids to accelerate the elimination of the medication through the kidneys and contact your doctor immediately.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount ingested.
If you forget to use Bilina
Do not apply an extra drop to the eye to make up for the missed dose.
If you have any other doubts about using this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, Bilina can cause side effects, although not everyone will experience them.
The frequency of the possible side effects listed below is defined using the following convention:
Very common (may affect more than 1 in 10 people)
Common (may affect up to 1 in 10 people)
Uncommon (may affect up to 1 in 100 people)
Rare (may affect up to 1 in 1,000 people)
Very rare (may affect up to 1 in 10,000 people)
Frequency not known (cannot be estimated from the available data)
The side effects observed with Bilina are:
Common side effects (affecting between 1 and 10 in 100 patients) may include:
- Reaction at the site of administration, such as burning/stinging in the eyes, eye irritation
Very rare side effects (affecting less than 1 in 10,000 patients) may include:
- A particular type of allergic reaction characterized by swelling of the lips, tongue, eyelids (angioedema) or other allergic reactions (anaphylaxis, hypersensitivity), such as hives and difficulty breathing
- Eye pain
- Conjunctivitis
- Eyelid swelling
- Eye swelling
- Eyelid inflammation
- Eye congestion
- Reaction at the site of administration, such as redness, itching in the eyes
- Skin irritation in the area in contact with the medication
- Hives
- Headache
- Palpitations
If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet.
Reporting side effects:
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect that is not listed in this leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Bilina
Keep out of sight and reach of children.
Do not use this medication after the expiration date stated on the container after CAD. The expiration date is the last day of the month indicated.
Discard 28 days after the first opening of the container.
Do not store above 25°C.
Medications should not be disposed of through wastewater or household waste. Deposit the containers and medications you no longer need at the pharmacy's SIGRE point. If you have any doubts, ask your pharmacist how to dispose of the containers and medications you no longer need. This will help protect the environment.
6. Package contents and additional information
Composition of Bilina
- The active ingredient is levocabastina. Each milliliter of Bilina contains 0.5 milligrams of levocabastina.
- The other components (excipients) are benzalkonium chloride, propylene glycol, anhydrous disodium phosphate, monosodium phosphate, hypromellose, polysorbate 80, disodium edetate, and water for injectable preparations.
Appearance of the product and package contents
Bilina is presented as a sterile eye drop suspension, white in color, in a 4 ml plastic dropper bottle.
Marketing authorization holder and manufacturer
Marketing authorization holder
Esteve Pharmaceuticals, S.A.
Passeig de la Zona Franca, 109
08038 Barcelona
Spain
Manufacturer
Famar, S.A.
Agiou Dimitriou 63
Alimos Attiki
17456 Greece
Date of the last revision of this leaflet: September 2020
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Average pharmacy price7.73 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BILINA 0.5 mg/ml EYE DROPS IN SUSPENSIONDosage form: EYE DROP, 0.54 mg levocabastine hydrochlorideActive substance: levocabastineManufacturer: Jntl Consumer Health (Spain) S.L.Prescription not requiredDosage form: EYEDROP, 0.5 mg/mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription requiredDosage form: EYEDROP, 1 mg/mlActive substance: olopatadineManufacturer: Tiedra Farmaceutica S.L.Prescription required
Online doctors for BILINA 0.5 mg/ml EYE DROPS IN SUSPENSION
Discuss questions about BILINA 0.5 mg/ml EYE DROPS IN SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















