
BEGLAN ACCUHALER 50 microgramos/inhalación, polvo para inhalación

Cómo usar BEGLAN ACCUHALER 50 microgramos/inhalación, polvo para inhalación
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Beglan Accuhaler 50 microgramos/inhalación, polvo para inhalación
salmeterol (xinafoato)
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Beglan Accuhaler y para qué se utiliza
- Qué necesita saber antes de empezar a usar Beglan Accuhaler
- Cómo usar Beglan Accuhaler
- Posibles efectos adversos
- Conservación de Beglan Accuhaler
- Contenido del envase e información adicional
1. Qué es Beglan Accuhaler y para qué se utiliza
Beglan Accuhaler pertenece al grupo de medicamentos denominados broncodilatadores.
Beglan Accuhaler está indicado para el alivio de los problemas de respiración en pacientes con asma o bronquitis crónica (EPOC).
2. Qué necesita saber antes de empezar a usar Beglan Accuhaler
No use Beglan Accuhaler
- si es alérgico al salmeterol o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Beglan Accuhaler:
- Si ha tenido alguna vez que dejar de tomar cualquier otro medicamento para el tratamiento de su enfermedad por problemas de alergia u otras razones.
- Si está en tratamiento por alguna alteración en el tiroides.
- Si está en tratamiento por tensión arterial elevada.
- Si está en tratamiento por problemas de corazón.
- Si tiene diabetes mellitus.
- Si tiene tendencia a presentar niveles bajos de potasio en sangre.
- Si está en tratamiento con ketoconazol (medicamento utilizado para el tratamiento de infecciones por hongos).
Si está utilizando Beglan Accuhaler para el tratamiento de su asma, su médico querrá verle con regularidad para comprobar sus síntomas. Acuda a su médico inmediatamente si:
- Su asma empeora.
- Tiene mayor dificultad para respirar.
- Nota más pitos.
- Tiene sensación de ahogo más a menudo.
- Tiene que utilizar su medicación de rescate con más frecuencia.
Si le ocurre cualquiera de las situaciones anteriormente mencionadas, no aumente el número de aplicaciones de Beglan Accuhaler (su situación respiratoria puede empeorar y enfermar gravemente). Acuda a su médico puesto que puede ser necesario que le cambien la medicación para el tratamiento de su asma.
Otros medicamentos y Beglan Accuhaler
Comunique a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Información importante sobre algunos de los componentes de BeglanAccuhaler
Uso en deportistas: este medicamento contiene salmeterol, que puede producir un resultado positivo en las pruebas de control de dopaje.
Este medicamento contiene lactosa. Puede provocar reacciones alérgicas en pacientes con alergia a la proteína de la leche de vaca. Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de usar este medicamento.
3. Cómo usar Beglan Accuhaler
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Recuerde usar su medicamento. ES MUY IMPORTANTE QUE SE UTILICE REGULARMENTE CADA DÍA. Esto le ayudará a mantenerse libre de síntomas a lo largo del día y de la noche.
Su médico le indicará la duración de su tratamiento con Beglan Accuhaler. No suspenda el tratamiento antes, aun cuando se encuentre mejor, a menos que su médico se lo indique.
Beglan Accuhaler solo debe utilizarse por vía inhalatoria y no debe utilizarse en niños menores de 4 años.
Si le han prescrito Beglan Accuhaler para su asma, debe continuar utilizando cualquier otro medicamento que esté tomando para controlar su asma. Éstos deben incluir un corticosteroide inhalado o en comprimidos. Continúe tomando las mismas dosis que antes, a no ser que su médico le diga lo contrario. Haga esto incluso si se siente mejor. No deje de tomar su corticosteroide inhalado (o en comprimidos) cuando comience a utilizar Beglan Accuhaler.
Es muy importante que siga las instrucciones de su médico sobre el número de veces y con qué frecuencia debe utilizar Beglan Accuhaler. Las instrucciones de uso se dan a continuación. Si tiene dificultades o no entiende las instrucciones pregunte a su médico o farmacéutico.
La dosis recomendada es:
Adultos
- Una inhalación (50 microgramos) dos veces al día. Si su médico se lo aconseja, puede incrementar esta dosis hasta dos inhalaciones (100 microgramos) dos veces al día.
Uso en niños y adolescentes
- Niños mayores de 4 años: una inhalación (50 microgramos) dos veces al día.
Si estima que la acción de Beglan es demasiado fuerte o débil, comuníqueselo a su médico o farmacéutico.
Instrucciones de uso:
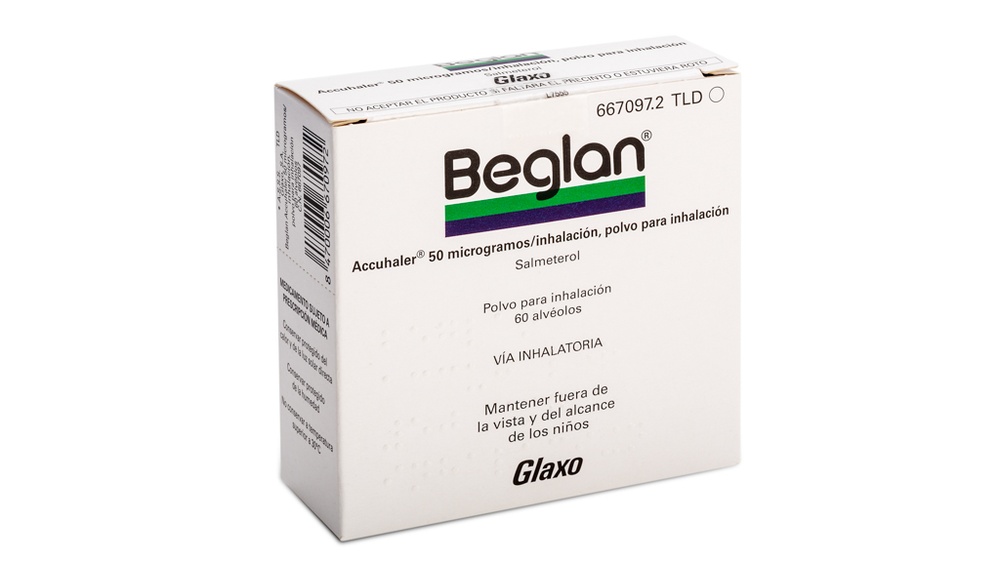
El dispositivo tiene dos posiciones: cerrado y abierto.
CERRADO
Al extraer del estuche por primera vez el dispositivo, estará cerrado.
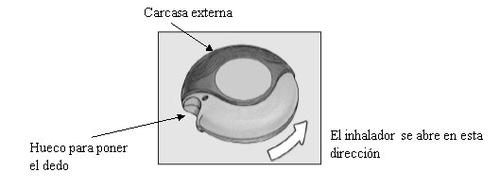
ABIERTO
El dispositivo contiene 60 dosis individuales del medicamento en forma de polvo. El indicador de dosis señala cuántas dosis quedan.
Cada dosis está precisamente medida y protegida higiénicamente. No se requiere ni mantenimiento ni rellenado.
El indicador de dosis en la parte superior del dispositivo señala cuántas dosis quedan. Los números de 5 a 0 aparecerán en ROJO para así advertir que quedan pocas dosis.
El manejo del dispositivo es fácil. Cuando se necesite una dosis, seguir las cuatro sencillas instrucciones siguientes:
- Abrir
- Deslizar
- Inhalar
- Cerrar
Funcionamiento del dispositivo
Al deslizar la palanca del dispositivo se abre un pequeño orificio en el aplicador bucal o boquilla y queda disponible una dosis ya preparada para ser inhalada. Cuando se cierra el dispositivo, la palanca automáticamente retrocede a su posición original quedando preparada para la siguiente dosis que necesite. La carcasa externa protege el dispositivo cuando no se utiliza.
- Abrir
Para abrir el dispositivo, coger con una mano la carcasa externa y colocar el dedo pulgar de la otra mano en el hueco reservado para ello. Empujar con el dedo alejándolo de usted hasta donde llegue.
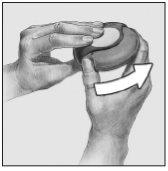
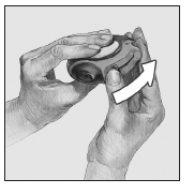
- Deslizar
Mantener el dispositivo con la boquilla hacia usted. Deslizar la palanca alejándola hasta donde llegue, se percibirá un sonido "click". El dispositivo está preparado para su uso. Cada vez que la palanca se echa hacia atrás, queda disponible una dosis para su inhalación. Esto lo muestra el contador de dosis. No manipular la palanca pues quedarían dosis disponibles que serían desperdiciadas.
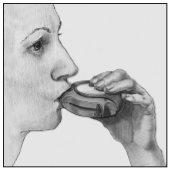
- Inhalar
Antes de empezar a inhalar una dosis, leer atentamente todo este apartado:
- Mantener el dispositivo alejado de la boca. Espirar (sacar el aire de los pulmones) lo que razonablemente se pueda (no hacerlo dentro del dispositivo).
- Colocar la boquilla en los labios. Inspirar (introducir aire en los pulmones) progresiva e intensamente - a través del dispositivo, no por la nariz.
- Sacar el dispositivo de la boca.
- Mantener la respiración unos 10 segundos o tanto tiempo como sea posible.
- Espirar lentamente.
- Es posible que no pueda saborear o sentir el polvo en su lengua, incluso si ha usado el dispositivo correctamente.
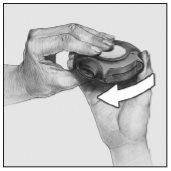
- Cerrar
Para cerrar el dispositivo, poner el dedo pulgar en el hueco reservado para ello y deslizarlo hacia usted, hasta donde llegue.
Al cerrar el dispositivo, se escuchará un golpe seco. La palanca automáticamente vuelve a su posición original y queda de nuevo colocada. El dispositivo está ahora preparado para volverlo a utilizar.
Si el médico ha prescrito dos inhalaciones, cerrar el dispositivo y repetir las instrucciones 1 a 4.
RECORDAR
Mantener el dispositivo seco.
Mantener el dispositivo cerrado cuando no se utilice.
No espirar dentro del dispositivo.
Deslizar la palanca únicamente cuando esté preparado para tomar una dosis.
No exceder la dosis indicada.
Mantener fuera del alcance de los niños.
Consulte a su médico o farmacéutico si tiene dudas.
Si usa más BeglanAccuhaler del que debe
Si ha utilizado Beglan Accuhaler más de lo que debe, puede notar: que el corazón late más rápidamente que de costumbre, dolor de cabeza, temblores, aumento de la presión sanguínea, niveles bajos de potasio en sangre, sensación de agitación y/o mareo. Consulte inmediatamente a su médico o farmacéutico o al Servicio de Información Toxicológica, teléfono: 91 562 04 20. Si la dosis tomada fuera muy elevada acudir al médico inmediatamente o al Servicio de Urgencias del hospital más próximo. Lleve este prospecto o el medicamento con usted.
Si olvidó usar BeglanAccuhaler
En caso de olvidar una dosis, no se preocupe. Inhale una dosis cuando se acuerde y luego siga como antes.
No tome una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Algunas personas pueden ser alérgicas a los medicamentos. En caso de tener alguno de los siguientes síntomas poco después de utilizar Beglan Accuhaler, INTERRUMPA la administración de este medicamento y avise al médico inmediatamente:
- Aparición súbita de pitos u opresión en el pecho.
- Inflamación de párpados, cara o labios.
- Erupción en la piel (habones) o urticaria en cualquier parte del cuerpo.
Algunas personas, particularmente aquellas que toman altas dosis de este tipo de medicamentos, pueden sentirse ocasionalmente un poco agitadas, tener dolor de cabeza o notar que su corazón late un poco más deprisa de lo normal, pero normalmente estos efectos desaparecen al continuar con el tratamiento. Si esta sensación continúa, comuníqueselo a su médico pero no interrumpa el tratamiento a no ser que él se lo diga.
A continuación se indican los efectos adversos asociados a salmeterol. Avise al médico si tiene alguno de los síntomas siguientes:
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 pacientes)
- Temblor y dolor de cabeza. Son características de este tipo de medicación y normalmente desaparecen con el tiempo. El temblor se produce más frecuentemente si usted recibe dosis superiores a 50 microgramos, dos veces al día.
- Palpitaciones, que normalmente desaparecen con el tiempo.
- Calambres musculares.
Efectos adversos poco frecuentes (pueden afectar hasta 1de cada 100pacientes)
- Reacciones de hipersensibilidad con erupción cutánea (Rash).
- Nerviosismo.
- Taquicardia (se produce más frecuentemente si usted recibe dosis superiores a 50 microgramos, dos veces al día).
Efectos adversos raros (pueden afectar hasta 1 de cada 1.000 pacientes)
- Hipopotasemia (niveles bajos de potasio en sangre).
- Insomnio.
- Mareos.
Efectos adversos muy raros (pueden afectar hasta 1 de cada 10.000 pacientes)
- Reacciones de hipersensibilidad incluyendo edema (hinchazón) y angioedema (reacción cutánea con enrojecimiento, hinchazón, picor y dificultad para respirar), broncoespasmo (contracción de los bronquios provocando dificultad para respirar) y shock anafiláctico (reacción alérgica grave).
- Hiperglucemia (niveles elevados de glucosa en sangre). Si tiene diabetes, puede necesitar controlar los niveles de azúcar con mayor frecuencia y posiblemente necesite un ajuste del tratamiento para la diabetes.
- Arritmias cardiacas, incluyendo fibrilación auricular, taquicardia supraventricular y extrasístoles (trastornos del ritmo del corazón).
- Irritación de garganta o faringe, broncoespasmo paradójico (estrechamiento de las paredes de los bronquios con disminución de la entrada de aire y dificultad para respirar).
- Náuseas.
- Artralgia (dolor de las articulaciones).
- Dolor torácico inespecífico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Beglan Accuhaler
Colocar el protector de la boquilla empujando firmemente y cerrando de un golpe para que la tapa quede en su sitio.
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 30ºC.
Conservar protegido del calor y de la luz solar directa.
Conservar protegido de la humedad.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Beglan Accuhaler
- El principio activo es 50 microgramos de salmeterol (como xinafoato) por cada aplicación.
- El otro componente es lactosa monohidrato (contiene proteínas de leche).
Aspecto del producto y contenido del envase
Beglan Accuhaler es polvo para inhalación. Cada dispositivo para inhalación contiene 60 alvéolos.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
GlaxoSmithKline, S.A.
P.T.M. C/ Severo Ochoa, 2
28760 Tres Cantos (Madrid)
Tel: +34 900 202 700
Responsable de la fabricación:
Glaxo Wellcome Production
Zone Industrielle N. 2
23 rue Lavoiser
27000 Evreux – Francia
Fecha de la última revisión de este prospecto:Septiembre 2020.
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia29.58 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BEGLAN ACCUHALER 50 microgramos/inhalación, polvo para inhalaciónForma farmacéutica: INHALACIÓN PULMONAR, 0,294 mg salmeterol hidroxinaftoatoPrincipio activo: salmeterolFabricante: Glaxosmithkline S.A.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 25 µg salmeterol hidroxinaftoato/aplicaciónPrincipio activo: salmeterolFabricante: Glaxosmithkline S.A.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 50 µgPrincipio activo: salmeterolFabricante: Glaxosmithkline S.A.Requiere receta
Médicos online para BEGLAN ACCUHALER 50 microgramos/inhalación, polvo para inhalación
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BEGLAN ACCUHALER 50 microgramos/inhalación, polvo para inhalación, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















