
ATROVENT NASAL 0,30 mg/ml SOLUCION PARA PULVERIZACION NASAL

Cómo usar ATROVENT NASAL 0,30 mg/ml SOLUCION PARA PULVERIZACION NASAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
PROSPECTO: INFORMACIÓN PARA EL USUARIO
Atrovent Nasal 0,30 mg/ml solución para pulverización nasal
Bromuro de ipratropio
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas, ya que puede perjudicarles.
- Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico.
Contenido del prospecto:
- Qué es Atrovent Nasal y para qué se utiliza
- Antes de usar Atrovent Nasal
- Cómo usar Atrovent Nasal
- Posibles efectos adversos
- Conservación de Atrovent Nasal
- Información adicional
1. Qué es Atrovent Nasal y para qué se utiliza
Atrovent Nasal pertenece al grupo de medicamentos denominados broncodilatadores anticolinérgicos, que actúan relajando la musculatura de los bronquios, facilitando así el paso del aire y por tanto, la respiración.
La rinitis es una inflamación de la mucosa de las fosas nasales. Atrovent Nasal es un medicamento que se utiliza para aliviar los síntomas de la rinorrea (goteo nasal) producida por la rinitis de causa alérgica y no alérgica.
2. Antes de usar Atrovent Nasal
No use AtroventNasal
- Si es alérgico (hipersensible) al bromuro de ipratropio o sustancias que son similares a ipratropio tales como atropina o a sus derivados, o a cualquiera de los demás componentes de Atrovent Nasal.
Tenga especial cuidado con AtroventNasal
- Si usted presenta predisposición a padecer glaucoma de ángulo estrecho (aumento de la presión interna del ojo), hiperplasia prostática (agrandamiento de la próstata) u obstrucción del cuello de la vejiga (si el conducto de la vejiga por el que se expulsa la orina está obstruido).
- Si usted padece fibrosis quística (una enfermedad que altera las secreciones de las glándulas mucosas y sudoríparas, afectando a varios órganos), puede ser más propenso a los trastornos de la motilidad gastrointestinal.
Tras la administración de Atrovent Nasal, pueden ocurrir reacciones de hipersensibilidad inmediata (reacciones alérgicas de rápida aparición) como urticaria, angioedema (hinchazón repentina de la piel o las mucosas), edema bucofaríngeo (hinchazón en la boca y la faringe) y anafilaxia (cuadro alérgico generalizado).
Se han comunicado casos aislados de complicaciones en los ojos, por ejemplo, midriasis (dilatación de la pupila), glaucoma de ángulo estrecho (aumento de la presión interna del ojo), dolor en el ojo por pulverización en los ojos de un aerosol de bromuro de ipratropio sólo o en combinación con un agonista ?2 adrenérgico (medicamentos que dilatan la musculatura de los bronquios, como por ejemplo salbutamol). Así pues, es preciso seguir estrictamente las instrucciones del médico sobre la administración correcta de Atrovent Nasal.
Un dolor o molestia del ojo, visión borrosa, halos visuales (luces difusas) o imágenes coloreadas, junto con enrojecimiento del ojo por congestión de la conjuntiva (el tejido interior del párpado) y edema de la córnea (pérdida de transparencia de la córnea), pueden constituir signos de glaucoma agudo de ángulo estrecho (aumento de la presión interna del ojo). En caso de observarse cualquier combinación de estos síntomas, debe notificarse inmediatamente al médico.
Uso de otros medicamentos
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente otros medicamentos, incluso los adquiridos sin receta.
No se ha descrito que el uso de Atrovent Nasal con otros fármacos, que habitualmente se prescriben para la rinitis perenne (rinitis que se presenta todo el año), tales como antihistamínicos (medicamentos utilizados en el tratamiento de las alergias), descongestivos o esteroides nasales (otros medicamentos utilizados para disminuir la inflamación de la mucosa de las fosas nasales), aumente la aparición de efectos secundarios. Aunque Atrovent Nasal pasa a la sangre sólo en una proporción mínima, existe la posibilidad de que se potencie su efecto si se administra junto con otros medicamentos anticolinérgicos, incluyendo aerosoles de bromuro de ipratropio para inhalación oral.
Embarazo
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
A pesar de que en los estudios preclínicos no se ha demostrado ningún riesgo, no se ha establecido la seguridad de Atrovent Nasal durante el embarazo. En caso de sospecha o confirmación de embarazo, deben valorarse los beneficios del tratamiento frente a los posibles riesgos para el feto.
Lactancia
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Se desconoce si el bromuro de ipratropio puede pasar a la leche materna. No obstante es improbable que el bromuro de ipratropio pase al lactante en cantidades importantes, especialmente cuando se administra por vía nasal. Sin embargo, debido a que muchos fármacos pueden pasar a la leche materna, se debe administrar con precaución a las mujeres en período de lactancia.
Conducción y uso de máquinas
No se dispone de estudios de los efectos sobre la capacidad para conducir y utilizar máquinas. Sin embargo, se advierte que pueden aparecer efectos adversos como mareos, dificultades del ojo para enfocar, dilatación de las pupilas y visión borrosa durante el tratamiento con Atrovent nasal. Por tanto, se recomienda precaución en la conducción y el uso de máquinas. Si los pacientes experimentan estos efectos deberían evitar realizar actividades potencialmente peligrosas como la conducción o la utilización de máquinas.
Atrovent Nasal contiene cloruro de benzalconio
Este medicamento contiene 0,25 mg de cloruro de benzalconio en cada ml.
El cloruro de benzalconio puede causar irritación o inflamación dentro de la nariz, especialmente cuando se usa durante periodos largos de tratamiento
3. Cómo usar Atrovent Nasal
Siga exactamente las instrucciones de administración de Atrovent Nasal indicadas por su médico. Consulte a su médico o farmacéutico si tiene dudas.
Su médico determinará la duración del tratamiento.
Si considera que la acción de Atrovent Nasal es demasiado fuerte o débil, comuníqueselo a su médico o farmacéutico.
La administración de Atrovent Nasal se debe adaptar a las necesidades individuales de cada paciente; los pacientes deben estar bajo supervisión médica durante el tratamiento.
La dosis normal es:
Adultos y adolescentes mayores de 12 años:2 pulverizaciones en cada fosa nasal, 2-3 veces al día.
Niños de6 a12 años:2 pulverizaciones en cada fosa nasal, 2 veces al día.
Sin embargo la dosis óptima puede variar según la respuesta individual de cada paciente y deberá ser establecida por el médico.
Modo de administración:
- Retirar el capuchón protector.
- Antes de utilizar el pulverizador por primera vez, presionar varias veces (aproximadamente 7 veces) hasta que se libere la primera nebulización (ver figura 1). Para activar la bomba, coger el frasco con los dedos pulgar, índice y medio.
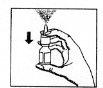 Asegurarse de que el frasco se dirige hacia arriba y se encuentra lejos de los ojos. Presionar con el pulgar firme y rápidamente el frasco (figura 1). La bomba estará ahora lista para el uso.
Asegurarse de que el frasco se dirige hacia arriba y se encuentra lejos de los ojos. Presionar con el pulgar firme y rápidamente el frasco (figura 1). La bomba estará ahora lista para el uso.
(figura 1)
Si la bomba no ha sido utilizada durante más de 24 horas, deberá activarse de nuevo con 1 ó 2 pulverizaciones.
- Antes de utilizar el pulverizador nasal, sonarse la nariz para limpiar las fosas nasales.
- Cerrar con un dedo una de las fosas nasales e inclinar ligeramente la cabeza hacia adelante. Con el frasco cogido como se indica en la figura 1, insertar el aplicador nasal del mismo en la otra fosa nasal (ver figura 2). Dirigir el aplicador hacia la parte posterior y externa de la nariz.

(figura 2)
- Activar la bomba una sola vez, presionando firme y rápidamente hacia arriba con el pulgar. Después de cada pulverización, aspirar (coger aire) profundamente y espirar (soltar aire) por la boca.
- Después de la pulverización y retirada del aplicador, inclinar la cabeza hacia atrás durante unos segundos para permitir que la pulverización difunda sobre la parte posterior de la nariz.
- Aplicar otra pulverización en la misma fosa nasal siguiendo el mismo procedimiento.
- Aplicar dos pulverizaciones en la otra fosa nasal siguiendo las mismas instrucciones.
- Volver a colocar el capuchón protector después de la utilización.
Si Atrovent Nasal se ha pulverizado accidentalmente sobre los ojos, lavarlos inmediatamente con agua fría.
Si el aplicador nasal se obstruye, retirar el capuchón protector. Colocar el aplicador nasal bajo el grifo de agua caliente durante aproximadamente un minuto. Secar el aplicador nasal, activar la bomba (operación 2) y colocar de nuevo el capuchón protector.
Si usa más Atrovent Nasal del que debiera
No se han descrito síntomas específicos de una sobredosis. Debido al amplio margen de seguridad y a que el uso de Atrovent Nasal es vía tópica, no son de esperar síntomas anticolinérgicos graves. Pueden producirse síntomas anticolinérgicos menores como sequedad de boca, trastornos de la acomodación visual y aumento de la presión cardiaca.
Si usted ha utilizado más Atrovent Nasal de lo que debe, consulte inmediatamente a su médico, farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20 indicando el medicamento y la cantidad ingerida.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Atrovent Nasal puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos frecuentes (se presentan en al menos 1 de cada 100 pacientes) son dolor de cabeza; irritación de garganta, sequedad nasal, sangrado nasal y molestias nasales.
Los efectos adversos poco frecuentes (se presentan al menos 1 de cada 1.000 pacientes) son reacciones anafilácticas (reacciones alérgicas graves), hipersensibilidad, mareo, halos visuales (luces difusas) o imágenes coloreadas asociadas a enrojecimiento de los ojos (glaucoma), aumento de la presión interna del ojo, dolor ocular, dilatación de las pupilas, trastorno de la acomodación visual (dificultad del ojo para enfocar), visión borrosa, halos visuales (luces difusas), enrojecimiento de los ojos, edema de córnea (hinchazón de la córnea), taquicardia supraventricular, fibrilación auricular, aumento de la frecuencia cardiaca, sequedad de boca y garganta, broncoespasmo (opresión en el pecho, pitos o falta de respiración), laringoespasmo (contracción de la laringe que produce dificultad para respirar), edema faríngeo (hinchazón de garganta), edema bucal (hinchazón de la boca), estomatitis (inflamación de la boca), erupción cutánea, angioedema (hinchazón de la cara, labios, boca, lengua o garganta que puede causar dificultad para tragar o respirar), retención de orina, trastornos de la motilidad gastrointestinal y náuseas.
Los efectos adversos raros (se presentan en al menos 1 de cada 10.000 pacientes) son palpitaciones, prurito (picor) y urticaria.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Atrovent Nasal
Mantener fuera del alcance y de la vista de los niños.
No utilice Atrovent Nasal después de la fecha de caducidad que aparece en el envase. La fecha de caducidad es el último día del mes que se indica.
No requiere condiciones especiales de conservación.
No utilizar después de 12 meses de abrir el envase por primera vez.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesite en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Información adicional
Composición de Atrovent Nasal
- El principio activo es bromuro de ipratropio. Cada ml de solución contiene 0,31 mg de bromuro de ipratropio monohidrato (equivalentes a 0,30 mg de bromuro de ipratropio anhidro). Una pulverización libera 21,7 microgramos de bromuro de ipratropio monohidrato, equivalentes a 21 microgramos de bromuro de ipratropio anhidro.
- Los demás componentes son: cloruro de sodio, cloruro de benzalconio, edetato de disodio, ácido clorhídrico, y agua purificada.
Aspecto del producto y contenido del envase
Envase con 15 ml (180 pulverizaciones) de solución para pulverización nasal.
Titular de la autorización de comercialización
Laboratorio STADA, S.L.
Frederic Mompou, 5
08960 Sant Just Desvern (Barcelona)España
Responsable de la fabricación
Istituto De Angeli S.r.l.
Localita i Prulli
50066 Reggello (Florencia)
o
BOEHRINGER INGELHEIM PHARMA GMBH & CO. KG
Birkendorfer Strasse, 65
D-88397 Biberach am der Riss
Alemania
Este prospecto ha sido aprobado en Julio 2013
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
- País de registro
- Precio medio en farmacia6.15 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ATROVENT NASAL 0,30 mg/ml SOLUCION PARA PULVERIZACION NASALForma farmacéutica: PRODUCTO USO NASAL, 12500 UI/gPrincipio activo: RetinolFabricante: Laboratorio Stada S.L.No requiere recetaForma farmacéutica: PRODUCTO USO NASAL, 850 mg cloruro sodico/ 100 mlPrincipio activo: variousFabricante: Laboratorios Cinfa S.A.No requiere recetaForma farmacéutica: PRODUCTO USO NASAL, 0,1 g azelastina hidrocloruro/100 mlPrincipio activo: azelastineFabricante: Cooper Consumer Health B.V.Requiere receta
Médicos online para ATROVENT NASAL 0,30 mg/ml SOLUCION PARA PULVERIZACION NASAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ATROVENT NASAL 0,30 mg/ml SOLUCION PARA PULVERIZACION NASAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











