
ATROVENT 20 microgramos SOLUCION PARA INHALACION EN ENVASE A PRESION


Cómo usar ATROVENT 20 microgramos SOLUCION PARA INHALACION EN ENVASE A PRESION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
PROSPECTO: INFORMACIÓN PARA EL USUARIO
Atrovent 20 microgramos solución para inhalación en envase a presión
bromuro de ipratropio
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Atrovent y para qué se utiliza
- Qué necesita saber antes de empezar a usar Atrovent
- Cómo usar Atrovent
- Posibles efectos adversos
- Conservación de Atrovent
- Contenido del envase e información adicional
1. Qué es Atrovent y para qué se utiliza
Atrovent pertenece al grupo de medicamentos denominados broncodilatadores anticolinérgicos, que actúan relajando la musculatura de los bronquios, facilitando así el paso del aire y por tanto, la respiración.
Atrovent pertenece al grupo de medicamentos denominados broncodilatadores por inhalación.
Atrovent se utiliza para el tratamiento de mantenimiento del broncoespasmo asociado a enfermedades pulmonares obstructivas crónicas (EPOC). La EPOC es una enfermedad de los pulmones donde hay una obstrucción al paso de aire por los bronquios y que causa dificultad para respirar.
2. Qué necesita saber antes de empezar a usar Atrovent
No use Atrovent
- Si es alérgico (hipersensible) al bromuro de ipratropio, a sustancias que son similares a ipratropio tales como atropina o sus derivados, o a cualquiera de los demás componentes de este medicamento (incluidos en la seccion 6).
- Si se presentan ataques agudos de tos, pitidos al respirar y dificultad respiratoria (broncoespasmo) que requieran una respuesta rápida.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Atrovent:
- Si padece fibrosis quística (una enfermedad que altera las secreciones de las glándulas mucosas y sudoríparas afectando a varios órganos), ya que puede ser más propenso a los trastornos de la motilidad gastrointestinal.
- Cuando se presente dificultad respiratoria aguda que empeora rápidamente. Debe consultar al médico inmediatamente.
- Podrían aparecer reacciones alérgicas inmediatas, tales como urticaria, angioedema (hinchazón de la cara, labios, boca, lengua o garganta que puede causar dificultad para tragar o respirar), erupción cutánea, tos, pitidos al respirar y dificultad respiratoria (broncoespasmo), hinchazón de boca y garganta (edema orofaríngeo) y cuadro alérgico generalizado (anafilaxia).
- Cuando padezca hiperplasia prostática (agrandamiento de la próstata) u obstrucción del cuello de la vejiga (si el conducto de la vejiga por el que se expulsa la orina está obstruido).
- Si tiene predisposición a padecer aumento de la presión interna del ojo (glaucoma de ángulo estrecho).
- Si pulveriza la solución en los ojos, pueden aparecer complicaciones en los ojos, como dilatación de la pupila, aumento de la presión interna del ojo (glaucoma de ángulo estrecho), dolor en el ojo, por lo que es preciso seguir las instrucciones del médico estrictamente para la administración. El riesgo de que la pulverización penetre en el ojo es limitado, dado que la inhalación se produce con una boquilla y está controlada manualmente.
- Si presenta una combinación de síntomas oculares, como dolor o molestia de los ojos, visión borrosa, halos visuales (luces difusas) o imágenes coloreadas junto con enrojecimiento de los ojos, pueden ser signos de glaucoma de ángulo estrecho, por lo que debe consultar a un médico de inmediato.
- Como con otros fármacos administrados por vía inhalatoria, Atrovent puede causar broncoespasmo paradójico, que puede poner en peligro su vida. En caso de producirse, se debe interrumpir el tratamiento de inmediato e informar al médico para sustituir por un tratamiento alternativo.
Otros medicamentos y Atrovent
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente o pudiera tener que utilizar otro medicamento.
No se recomienda la administración simultánea por un periodo largo de tiempo de Atrovent con otros fármacos anticolinérgicos.
Los beta-adrenérgicos (p. ej. salbutamol) y los derivados de la xantina (p. ej. teofilina) son otros medicamentos broncodilatadores y pueden potenciar el efecto dilatador de los bronquios. Atrovent puede acentuar los efectos anticolinérgicos de otros fármacos.
Atrovent se puede administrar conjuntamente con otros fármacos comúnmente utilizados en el tratamiento de la enfermedad pulmonar obstructiva crónica (EPOC), incluyendo medicamentos beta-adrenérgicos, metilxantinas (p. ej. teofilina), esteroides y cromoglicato disódico, sin aparición de interacciones perjudiciales.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No se ha establecido la seguridad del medicamento durante el embarazo. Debe valorarse cuidadosamente el beneficio de la utilización, frente al riesgo potencial para el feto, por lo que deben observarse las precauciones habituales en el uso de medicamentos durante este periodo.
Se desconoce si bromuro de ipratropio puede pasar a la leche materna. No obstante, es poco probable que pueda ser ingerido por el lactante en cantidades significativas, especialmente porque el preparado se administra por vía inhalatoria. Sin embargo, se debe administrar con precaución a las mujeres en periodo de lactancia.
Conducción y uso de máquinas
No se dispone de estudios de los efectos sobre la capacidad para conducir y utilizar máquinas. Sin embargo, se advierte que pueden aparecer efectos adversos como mareos, dificultades del ojo para enfocar, dilatación de las pupilas y visión borrosa durante el tratamiento con Atrovent. Por tanto, se recomienda precaución en la conducción y el uso de máquinas.
Atrovent contiene etanol
Este medicamento contiene unos 8 mg de alcohol (etanol) en cada pulverización.
La cantidad en cada pulverización de este medicamento es equivalente a menos de 1 ml de cerveza o 1 ml de vino. La pequeña cantidad de alcohol que contiene este medicamento no produce ningún efecto perceptible..
3. Cómo usar Atrovent
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Recuerde usar su medicamento.
El uso correcto de la solución para inhalación es decisivo para el éxito del tratamiento.
Antes de la primera utilización de cada envase se accionará 1 ó 2 veces el aparato dosificador después de haberlo agitado.
Antes de cada aplicación se seguirán las siguientes instrucciones:

(fig. 1)
- Retirar el capuchón protector.
- Realizar una espiración (expulsión de aire por la boca) profunda.
- Coger el inhalador con la mano en la forma que se indica en la figura 1 y apretar firmemente la boquilla con los labios. La flecha y la base del recipiente deben dirigirse hacia arriba.
- Realizar una inspiración (toma de aire) lo más enérgica posible y, al mismo tiempo, presionar con fuerza sobre la base del recipiente, con lo que se libera una pulverización de solución para inhalación. Contener la respiración durante algunos segundos, retirar luego la boquilla y espirar (soltar el aire) lentamente. Si no puede inspirar profundamente a causa de la dificultad para respirar, puede facilitar la respiración aplicando una pulverización de solución para inhalación en la boca antes de la aplicación del medicamento.
- Una vez usado, volver a colocar el capuchón protector.
- En caso de no haber utilizado el inhalador durante un periodo de 3 días, se tendrá que accionar la válvula una vez más.
Como el recipiente no es transparente, es imposible ver cuando está vacío.
El envase proporcionará 200 dosis. Cuando se haya utilizado el número de dosis indicadas en el etiquetado (normalmente después de 3 semanas si se ha utilizado como se recomienda) puede parecer que el envase contiene todavía una pequeña cantidad de líquido. Aún así, el inhalador se debe sustituir para asegurar que está usted obteniendo la cantidad de medicamento correcta en cada inhalación.
Limpie la boquilla al menos una vez por semana. Es importante mantener la boquilla siempre limpia para asegurar que el medicamento no se acumula y bloquea el spray. Para lavarla, retire el capuchón protector y el envase de la boquilla. Enjuague pasando agua caliente a través de la boquilla hasta la eliminación de cualquier resto de medicamento o suciedad visibles (figura 2).
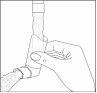
(fig. 2)
Después de limpiar, sacudir la boquilla y dejar secar al aire sin utilizar ningún sistema de calefacción. Una vez la boquilla está seca, colocar de nuevo el envase y el capuchón protector (figura 3).
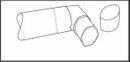
(fig. 3)
ADVERTENCIA: La boquilla de plástico se ha diseñado especialmente para su utilización con Atrovent, con el fin de asegurar que siempre se obtiene la cantidad correcta del preparado. La boquilla nunca se debe utilizar con otra solución para inhalación en envase a presión, ni tampoco Atrovent se debe utilizar con otra boquilla distinta a la que se proporciona con el producto.
Si estima que la acción de Atrovent es demasiado fuerte o débil, comuníqueselo a su médico o farmacéutico.
Atrovent 20 microgramos solución para inhalación en envase a presión se administra por vía inhalatoria.
La dosificación (número de inhalaciones al día) debe adaptarse a las necesidades de cada paciente. Para adultos y niños mayores de 6 años, se recomienda la siguiente dosificación:
2 inhalaciones (equivalente a 40 microgramos de bromuro de ipratropio anhidro), 4 veces al día.
Dado que la necesidad de dosis cada vez mayores sugiere que pueda ser necesario un tratamiento con otro fármaco de manera adicional, por lo general, no debe superarse una dosis diaria total de 12 inhalaciones (240 microgramos de bromuro de ipratropio anhidro).
Debe consultar al médico si no consigue una mejoría importante o si su estado empeora, con el fin de determinar si es necesario un nuevo tratamiento. Asimismo, debe consultar al médico sin tardanza en caso de dificultad importante para respirar o cuando la dificultad para respirar se agrave rápidamente.
En los niños, Atrovent sólo debe administrarse por indicación del médico y siempre bajo la vigilancia de un adulto.
Su médico le indicará la duración de su tratamiento con Atrovent. No suspenda el tratamiento antes, ya que el médico es la persona indicada para darle las instrucciones precisas.
Si usa más Atrovent del que debe
Podrían aparecer síntomas tales como sequedad de boca, trastornos de la acomodación visual (dificultad del ojo para enfocar) y taquicardia (aumento de la frecuencia cardiaca).
Si ha utilizado más Atrovent de lo que debe, consulte inmediatamente a su médico o a su farmacéutico, o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Atrovent
No use una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos frecuentes (se presentan en al menos 1 de cada 100 pacientes) con la administración de Atrovent son dolor de cabeza, mareo, tos, irritación de garganta, náuseas, sequedad de boca y trastornos de la motilidad gastrointestinal (p. ej.: cambio en el hábito intestinal, reflujo gastroesofágico, dispepsia (indigestión)).
Los efectos adversos poco frecuentes (se presentan en al menos 1 de cada 1.000 pacientes) son hipersensibilidad, reacción anafiláctica (reacción alérgica grave), visión borrosa, midriasis (dilatación de la pupila), aumento de la presión interna del ojo, halos visuales (luces difusas) o imágenes coloreadas asociadas a enrojecimiento de los ojos (glaucoma), dolor ocular, halos visuales (luces difusas), enrojecimiento de los ojos, edema de córnea (hinchazón de la córnea), palpitaciones, taquicardia supraventricular, estreñimiento, diarrea, vómitos, estomatitis (inflamación de la boca), edema bucal (hinchazón de la boca), erupción, prurito (picor), angioedema (hinchazón de la cara, labios, boca, lengua o garganta que puede causar dificultad para tragar o respirar) y retención de orina.
Los efectos adversos raros (se presentan en al menos 1 de cada 10.000 pacientes) son broncoespasmo (opresión en el pecho, pitos o falta de respiración), broncoespasmo paradójico (estrechamiento de las paredes de los bronquios debido a la propia inhalación), contracción de laringe, edema faríngeo (hinchazón de garganta), sequedad de garganta, trastorno de la acomodación visual (dificultad del ojo para enfocar), urticaria, aumento de la frecuencia cardiaca y fibrilación auricular.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Atrovent
Mantener este medicamento fuera de la vista y del alcance de los niños.
El envase presurizado no debe ser perforado ni expuesto a temperaturas superiores a 50 ºC.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y en la etiqueta del inhalador después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesite en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Atrovent
- El principio activo es bromuro de ipratropio. Cada pulverización contiene 21 microgramos de bromuro de ipratropio monohidrato (equivalentes a 20 microgramos de bromuro de ipratropio anhidro).
- Los demás componentes son ácido cítrico anhidro, agua purificada, etanol (15 % p/p) y HFA 134a (1,1,1,2-tetrafluoroetano).
Este medicamento contiene gases fluorados de efecto invernadero.
Cada inhalador contiene 12,33 g de HFA 134a que corresponden a 0,01763 toneladas de CO2 equivalente (potencial de calentamiento global PCG = 1430).
Aspecto del producto y contenido del envase
Atrovent es una solución para inhalación en envase a presión, que se presenta en envases de 10 ml de solución (200 pulverizaciones) para inhalación por vía oral.
Los propelentes clorofluorocarbonados pueden dañar la capa de ozono de la atmósfera. Atrovent carece de este tipo de propelentes y sólo contiene el propelente no clorofluorocarbonado HFA 134a, que no contribuye al agotamiento de la capa de ozono.
Titular de la autorización de comercialización
Boehringer Ingelheim España, S.A.
Prat de la Riba, 50
08174 Sant Cugat del Vallès (Barcelona)
España
Responsable de la fabricación
Boehringer Ingelheim Pharma GmbH & Co. KG
Binger Strasse, 173
55216 Ingelheim am Rhein
Alemania
Fecha de la última revisión de este prospecto: Enero 2025
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
- País de registro
- Precio medio en farmacia4.46 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ATROVENT 20 microgramos SOLUCION PARA INHALACION EN ENVASE A PRESIONForma farmacéutica: INHALACIÓN PULMONAR, 0,0040 g/aerosolPrincipio activo: Ipratropio bromuroFabricante: Laboratorio Aldo Union S.L.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 250 µgPrincipio activo: Ipratropio bromuroFabricante: Boehringer Ingelheim Espana S.A.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 500 µgPrincipio activo: Ipratropio bromuroFabricante: Boehringer Ingelheim Espana S.A.Requiere receta
Médicos online para ATROVENT 20 microgramos SOLUCION PARA INHALACION EN ENVASE A PRESION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ATROVENT 20 microgramos SOLUCION PARA INHALACION EN ENVASE A PRESION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















