

АТРОПИН Агеттант 0,1 мг/мл раствор для инъекций в предварительно заполненном шприце

Спросите врача о рецепте на АТРОПИН Агеттант 0,1 мг/мл раствор для инъекций в предварительно заполненном шприце

Инструкция по применению АТРОПИН Агеттант 0,1 мг/мл раствор для инъекций в предварительно заполненном шприце
Введение
Прошпект: Информация для пользователя
АТРОПИН СУЛЬФАТ АГЕТТАНТ 0,1мг/мл, раствор для инъекций в предварительно заполненном шприце
Атропин Сульфат
Прочитайте внимательно весь прошпект перед началом использования этого лекарства, поскольку он содержит важную информацию для вас.
- Сохраните этот прошпект, поскольку вам может потребоваться перечитать его.
- Если у вас есть какие-либо сомнения, проконсультируйтесь с вашим врачом или фармацевтом.
- Это лекарство было назначено только вам, и его не следует давать другим людям, даже если у них такие же симптомы, как у вас, поскольку оно может нанести им вред.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом или фармацевтом, даже если это побочные эффекты, которые не указаны в этом прошпекте. См. раздел 4.
Название этого лекарства - Атропин Агеттант 0,1 мг/мл раствор для инъекций в предварительно заполненном шприце, которое будет упоминаться как Атропин Агеттант на протяжении всего прошпекта.
Содержание прошпекта:
- Что такое Атропин Сульфат Агеттант и для чего он используется
- Что вам нужно знать перед началом использования Атропин Агеттант
- Как использовать Атропин Агеттант
- Возможные побочные эффекты
- Хранение Атропин Агеттант
- Содержание упаковки и дополнительная информация
1. Что такое Атропин Агеттант и для чего он используется
Атропин относится к классу лекарств, называемых антихолинергическими. Антихолинергическое средство - это вещество, которое блокирует нейротрансмиттер ацетилхолин в центральной и периферической нервной системе. Он используется в ситуациях экстренной необходимости, когда сердце бьется слишком медленно, в качестве антидота для случаев отравления инсектицидами органофосфорными или нервно-паралитическим газом, и для случаев отравления грибами.
Он может использоваться как часть предварительной медикаментозной подготовки перед общей анестезией. Также он может использоваться для предотвращения побочных эффектов других лекарств, используемых для устранения эффектов миорелаксантов после хирургического вмешательства.
Атропин 0,1 мг/мл раствор для инъекций в предварительно заполненном шприце используется для лечения взрослых и детей с рождения с весом тела более 3 кг.
2. Что вам нужно знать перед началом использования Атропин Агеттант
Не используйте Атропин Агеттант:
- если вы аллергичны (гиперчувствительны) к атропину или любому другому компоненту этого лекарства (перечисленному в разделе 6);
- у вас есть проблемы с мочеиспусканием;
- у вас повышенное давление в глазу (глаукома);
- вы страдаете заболеванием пищевода (ахалазией пищевода), блокировкой кишечника (паралитическим илеусом) или острым формой колического расширения (токсическим мегаколоном).
Эти противопоказания не действуют в случае потенциально смертельных экстренных ситуаций.
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом перед началом использования Атропин Агеттант, если у вас:
- гипертиреоз;
- простатическая гиперплазия;
- сердечная недостаточность;
- печеночная или почечная недостаточность;
- некоторые сердечные заболевания;
- гастрит, такой как пилорический стеноз;
- хронический бронхит;
- лихорадка;
- если вы ребенок или пожилой человек;
- тяжелая миастения (мышечная слабость);
- изжога (рефлюкс).
Использование Атропин Агеттант с другими лекарствами
Сообщите вашему врачу или фармацевту, если вы принимаете, недавно принимали или можете принимать любое другое лекарство:
- трициклические антидепрессанты;
- некоторые антигистаминные препараты;
- лекарства для лечения болезни Паркинсона;
- фенотиязин, клозапин или нейролептические препараты (для психических заболеваний);
- хинидин или дисопирамид (для сердечных заболеваний);
- спазмолитики (для синдрома раздраженного кишечника).
Беременность и лактация
Беременность
Ограниченные данные об использовании атропина у беременных женщин указывают на то, что нет негативных эффектов на беременность и здоровье плода. Атропин проникает через плаценту. Внутривенное введение атропина во время беременности или в конце срока может вызвать более быстрый сердечный ритм у плода и матери. Это лекарство должно использоваться во время беременности только после тщательного рассмотрения пользы и рисков лечения.
Лактация
Небольшие количества атропина могут проникать в грудное молоко и оказывать эффекты на ребенка. Атропин может ингибировать производство грудного молока. Ваш врач оценит пользу грудного вскармливания и пользу лечения. Если решено продолжить лечение, грудное вскармливание должно быть прервано. Однако, если решено продолжить грудное вскармливание во время лечения, ваш врач проведет дополнительные обследования ребенка.
Если вы беременны или кормите грудью, считаете, что можете быть беременной или планируете стать беременной, проконсультируйтесь с вашим врачом или фармацевтом перед использованием этого лекарства.
Вождение и использование машин
Введение атропина может вызвать путаницу или размытое зрение. Не следует управлять транспортными средствами или использовать машины после получения инъекции.
Атропин Агеттант содержит натрий
Это лекарство содержит менее 1 ммоль натрия (23 мг) на дозу, что означает, что оно практически не содержит натрия.
3. Как использовать Атропин Агеттант
Ваш врач решит, какая доза правильна для вас, и как и когда будет введена инъекция. В случае сомнений проконсультируйтесь с вашим врачом.
Рекомендуемая доза:
Как предварительное лечение перед анестезией
Внутривенное введение (IV) непосредственно перед хирургическим вмешательством; при необходимости возможно внутримышечное введение за 30-60 минут до операции. Взрослые: 0,3-0,6 мг IV
Дети: 0,01-0,02 мг/кг, с коррекцией дозы в зависимости от реакции и толерантности пациента (максимально 0,6 мг на дозу).
Для устранения эффектов миорелаксантов:
Взрослые: 0,6-1,2 мг IV с неостигмином.
Дети: 0,02 мг/кг IV
В случае низкой частоты сердечных сокращений, блокады сердца или остановки сердца:
Взрослые:
- Синусовая брадикардия (низкая частота пульса): 0,5 мг IV, каждые 2-5 минут до достижения желаемой частоты сердечных сокращений.
- Атриовентрикулярная блокада (блокада передачи сокращения между предсердием и желудочком): 0,5 мг IV, каждые 3-5 минут (максимально 3 мг).
Дети: 0,02 мг/кг IV в виде единой дозы (максимальная доза 0,6 мг).
Как антидот для отравления органофосфорными соединениями (инсектицидами или нервно-паралитическим газом), антихолинэстеразами или отравления грибами мускаринового типа:
Взрослые: 0,5-2 мг IV, может повторяться через 5 минут и затем каждые 10-15 минут по мере необходимости.
Дети: 0,02 мг/кг, может повторяться несколько раз до исчезновения симптомов.
Возможно, что другие формы этого лекарства будут более подходящими, если требуется доза выше 0,5 мг.
Использование у детей
Атропин используется для лечения детей с рождения с весом более 3 кг.
Если у вас есть какие-либо другие вопросы об использовании этого лекарства, проконсультируйтесь с вашим врачом или фармацевтом.
Эта инъекция будет введена врачом или медсестрой, поэтому маловероятно, что вы получите слишком большую дозу атропина. Если вы считаете, что вам была введена слишком большая доза атропина, чувствуете, что ваше сердце бьется слишком быстро, вы дышите быстро, у вас высокая температура, вы чувствуете беспокойство, путаницу, имеете галлюцинации или теряете координацию, сообщите об этом человеку, который ввел инъекцию.
4. Возможные побочные эффекты
Как и все лекарства, это лекарство может вызывать побочные эффекты, хотя не все люди испытывают их.
Побочные эффекты зависят от дозы, которую вы получаете, и обычно исчезают после прекращения лечения.
Редко может произойти аллергическая реакция. Это может вызвать кожные высыпания, сильный зуд, шелушение кожи, отек лица (особенно вокруг губ и глаз), чувство сдавления в горле и затруднение дыхания или глотания, температуру, обезвоживание, шок и обморок. Все эти побочные эффекты очень серьезны. Сообщите вашему врачу немедленно, если вы испытываете любой из этих побочных эффектов. Вам может потребоваться срочная медицинская помощь.
Очень частые побочные эффекты (которые могут возникать более чем у 1 из 10 человек)
- нарушения зрения (расширение зрачков, трудности с фокусировкой, размытое зрение, чувствительность к свету);
- уменьшение бронхиальной секреции;
- сухость во рту (трудности с глотанием и речью, чувство жажды);
- запор и изжога (рефлюкс);
- уменьшение секреции желудочной кислоты;
- потеря вкуса;
- тошнота;
- рвота;
- чувство распирания;
- отсутствие пота;
- сухость кожи;
- крапивница;
- сыпь.
Частые побочные эффекты (которые могут возникать не более чем у 1 из 10 человек)
- возбуждение (особенно при более высоких дозах);
- потеря координации (особенно при более высоких дозах);
- путаница (особенно при более высоких дозах);
- галлюцинации (особенно при более высоких дозах),
- повышение температуры;
- определенные сердечные расстройства (учащенное сердцебиение, нерегулярное сердцебиение, временная замедление сердечного ритма);
- покраснение;
- затруднение мочеиспускания.
Редкие побочные эффекты (которые могут возникать не более чем у 1 из 100 человек)
- психотические реакции.
Очень редкие побочные эффекты (которые могут возникать не более чем у 1 из 1000 человек)
- аллергические реакции;
- эпилептические приступы (судороги);
- сономоленция.
Очень редкие побочные эффекты (которые могут возникать не более чем у 1 из 10 000 человек)
- тяжелая аллергическая реакция;
- нерегулярное сердцебиение, включая фибрилляцию желудочков;
- грудная боль;
- пик артериального давления.
Частота не известна (не может быть оценена из доступных данных)
- головная боль;
- беспокойство;
- неустойчивая походка и проблемы с равновесием;
- бессонница.
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, проконсультируйтесь с вашим врачом или фармацевтом, даже если это возможные побочные эффекты, которые не указаны в этом прошпекте. Вы также можете сообщить о побочных эффектах через Систему испанской фармаковигиланции лекарственных средств для человека: www.notificaRAM.es. Сообщая о побочных эффектах, вы можете способствовать предоставлению более полной информации о безопасности этого лекарства.
5. Хранение Атропин Агеттант
Храните это лекарство в недоступном для детей месте.
Не используйте это лекарство после даты истечения срока годности, указанной на упаковке, шприце и блистере. Дата истечения срока годности - последний день месяца, указанного.
Это лекарство не требует специальных условий хранения.
Не используйте это лекарство, если вы заметили видимые признаки повреждения.
Лекарства не должны выбрасываться в канализацию или мусор. Спросите вашего фармацевта, как утилизировать упаковку и лекарства, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
Состав Атропин Агеттант
- Активное вещество - Атропин Сульфат:
Каждый мл раствора для инъекций содержит 0,1 мг Атропин Сульфат моногидрат, что эквивалентно 0,083 мг атропина.
Каждый шприц 5 мл содержит 0,5 мг Атропин Сульфат моногидрат, что эквивалентно 0,415 мг атропина.
- Другие компоненты -
Хлорид натрия, концентрированная соляная кислота (для коррекции pH), вода для инъекций.
Внешний вид продукта Атропин Агеттант и содержание упаковки.
Это лекарство представляет собой прозрачный и бесцветный раствор для инъекций в предварительно заполненном шприце из полипропилена объемом 5 мл.
Упаковки по 10, 12 и 20 шприцев.
Возможно, что не все размеры упаковок будут доступны.
Владелец разрешения на маркетинг и производитель
Владелец разрешения на маркетинг:
LABORATOIRE AGUETTANT
1, RUE ALEXANDER FLEMING
69007 LYON
ФРАНЦИЯ
Производитель:
LABORATOIRE AGUETTANT
1, rue Alexander Fleming
69007 LYON
Франция
LABORATOIRE AGUETTANT
Lieu-Dit “Chantecaille ”
07340 CHAMPAGNE
Франция
Дата последней ревизии этого прошпекта: Декабрь 2021
Подробная информация о этом лекарстве доступна на сайте Агентства по лекарственным средствам и медицинским изделиям (AEMPS). http//www.aemps.gob.es
Эта информация предназначена только для медицинских специалистов:
Предварительно заполненный шприц предназначен для одного пациента. Утилизируйте шприц после использования. Не повторно использовать.
Содержимое неповрежденного и неоткрытого блистера стерильно и не должно открываться до использования.
Продукт должен визуально осматриваться на наличие частиц и изменений цвета перед введением. Должно использоваться только прозрачное, бесцветное и не содержащее частиц или осадков решение.
Продукт не должен использоваться, если целостность защитного покрытия шприца (пластиковая оболочка до колпачка) нарушена.
Внешняя поверхность шприца стерильна до тех пор, пока блистер не будет открыт.
- Извлеките предварительно заполненный шприц из стерильного блистера.
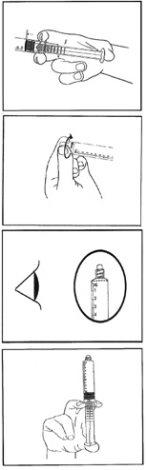
- Нажмите на поршень, чтобы освободить пробку.
- Поверните колпачок, чтобы снять его и нарушить целостность защитного покрытия.
- Проверьте, что защитное покрытие шприца (пластиковая оболочка до колпачка и защитное покрытие под колпачком) полностью удалено. В противном случае, снова наденьте колпачок и поверните его.
- Аккуратно нажмите на поршень, чтобы выпустить воздух.
- Подключите шприц к устройству для доступа к сосудам или игле. Нажмите на поршень, чтобы ввести необходимый объем.
Необходимый размер иглы для использования с шприцем - 23-20 для внутривенного введения и 23-21 для внутримышечного введения.
- Страна регистрации
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги АТРОПИН Агеттант 0,1 мг/мл раствор для инъекций в предварительно заполненном шприцеФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 0,1 мг/млАктивное вещество: atropineПроизводитель: Accord Healthcare S.L.U.Требуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 0.2 мг/млАктивное вещество: atropineПроизводитель: Laboratoire AguettantТребуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 1 мг/млАктивное вещество: atropineПроизводитель: Noridem Enterprises LimitedТребуется рецепт
Аналоги АТРОПИН Агеттант 0,1 мг/мл раствор для инъекций в предварительно заполненном шприце в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог АТРОПИН Агеттант 0,1 мг/мл раствор для инъекций в предварительно заполненном шприце в Польша
Аналог АТРОПИН Агеттант 0,1 мг/мл раствор для инъекций в предварительно заполненном шприце в Украина
Врачи онлайн по АТРОПИН Агеттант 0,1 мг/мл раствор для инъекций в предварительно заполненном шприце
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на АТРОПИН Агеттант 0,1 мг/мл раствор для инъекций в предварительно заполненном шприце – по решению врача и с учетом местных правил.














