ADVATE 500 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar ADVATE 500 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el usuario
ADVATE250UI polvo ydisolvente para solución inyectable
ADVATE500UI polvo ydisolvente para solución inyectable
ADVATE1000UI polvo ydisolvente para solución inyectable
ADVATE1500UI polvo ydisolvente para solución inyectable
Octocog alfa (factor VIII humano de coagulación recombinante)
Lea todo el prospecto detenidamente antes de empezar ausar el medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos sintomas, ya que puede perjudicarles.
- Si experimenta cualquier tipo de efecto adverso, consulte a su medico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es ADVATE y para qué se utiliza
- Qué necesita saber antes de empezar a usar ADVATE
- Cómo usar ADVATE
- Posibles efectos adversos
- Conservación de ADVATE
- Contenido del envase e información adicional
1. Qué es ADVATE y para qué se utiliza
ADVATE contiene el principio activo octocog alfa, factor VIII humano de coagulación producido por tecnología de ADN recombinante. El factor VIII es necesario para coagular la sangre y parar las hemorragias. En pacientes con hemofilia A el factor VIII falta o no funciona correctamente (ausencia hereditaria de factor VIII).
ADVATE se utiliza para el tratamiento y la prevención de hemorragias en pacientes de todos los grupos de edad que padecen hemofilia A (un trastorno hemorrágico hereditario provocado por la ausencia de factor VIII).
ADVATE se prepara sin añadir ninguna proteína derivada humana o animal en todo el proceso de fabricación.
2. Qué necesita saber antes de empezar a usar ADVATE
No use ADVATE
- si es alérgico (hipersensible) al octocog alfa o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- si es alérgico a las proteínas del ratón o del hámster
Si no está seguro si es alérgico, consulte con su médico.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar ADVATE. Informe a su médico si ha sido tratado previamente con medicamentos que contienen factor VIII, especialmente si desarrolló inhibidores ya que puede existir un riesgo elevado de que esto vuelva a suceder. Los inhibidores son anticuerpos que bloquean el factor VIII que reducen la eficacia de ADVATE para prevenir el control del sangrado. El desarrollo de inhibidores es una complicación conocida en el tratamiento de la hemofilia A. Si su hemorragia no llega a controlarse con ADVATE, consulte a su médico inmediatamente.
Existe un riesgo escasode que pueda experimentar una reacción anafiláctica (una reacción alérgica repentina grave) a ADVATE. Debe ser consciente de que los primeros síntomas de una reacción alérgica son sarpullido, picor, ronchas, picor generalizado, hinchazón de los labios y lengua, dificultad para respirar, jadeos, opresión en el pecho, sensación de malestar general y mareos. Estos síntomas pueden ser un aviso de shock anafiláctico, que también puede producir mareos graves, pérdida de consciencia y dificultades graves para respirar.
Si experimenta cualquiera de estos síntomas, interrumpa inmediatamente la administración del medicamento y consulte con un médico. Los síntomas graves, incluyendo la dificultad para respirar y (casi) desmayos requieren un tratamiento de emergencia rápido.
Pacientes que desarrollan inhibidores del Factor VIII
La formación de inhibidores (anticuerpos) es una complicación conocida que puede producirse durante el tratamiento con todos los medicamentos compuestos por factor VIII. Estos inhibidores, especialmente en grandes cantidades, impiden que el tratamiento funcione correctamente, por lo que se les supervisará cuidadosamente a usted y a su hijo por si desarrollan dichos inhibidores. Si su hemorragia o la de su hijo no se está controlando con ADVATE, consulte a su médico inmediatamente.
Niños y adolescentes
Las advertencias y precauciones indicadas se aplican a adultos y niños (de 0 a 18 años de edad).
Uso de ADVATE con otros medicamentos
Informe a su médico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Embarazo ylactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su medico antes de utilizar este medicamento.
Conducción yuso de máquinas
El tratamiento con ADVATE no tiene influencia sobre la capacidad para conducir y utilizar maquinaria.
ADVATE contiene sodio
Este medicamento contiene 0,45 mmol de sodio (10 mg) por vial, lo que se debe tener en cuenta en pacientes con dietas pobres en sodio.
Aplicación incorrecta de ADVATE
La aplicación incorrecta (la inyección en la arteria o fuera de la vena) se debe evitar ya que pueden producirse reacciones leves y a corto plazo en el lugar de la inyección, como cardenales y enrojecimiento.
3. Cómo usar ADVATE
El tratamiento con ADVATE se iniciará por un médico con experiencia en el tratamiento de pacientes con hemofilia A.
Su médico calculará su dosis de ADVATE (en unidades internacionales o UI) en función de su estado, su peso corporal y de si va a ser utilizado para la prevención o para el tratamiento de hemorragias. La frecuencia de administración dependerá de cómo actúe ADVATE. La terapia sustitutiva con ADVATE es normalmente un tratamiento de por vida.
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
Prevención de hemorragias
La dosis habitual de octocog alfa es de 20 a 40 UI por kg de peso corporal, administrado cada 2 ó 3 días. Sin embargo, en algunos casos, especialmente en los pacientes más jóvenes, pueden requerirse la administración más frecuente de las inyecciones o dosis mayores.
Tratamiento de hemorragias
La dosis de octocog alfa se calcula en función de su peso corporal y de los niveles de factor VIII que se quieran conseguir. Los niveles de factor VIII a alcanzar dependerán de la gravedad y de la localización de la hemorragia.
Dosis (UI) = peso corporal (kg) x aumento de Factor VIII deseado (% del normal) x 0,5
Si tiene la impresión de que el efecto de ADVATE es insuficiente, consulte con su médico.
Su médico realizará los análisis de laboratorio apropiados para asegurarse de que tiene los niveles adecuados de factor VIII. Esto es especialmente importante si se va a someter a una operación importante.
Uso en niños y adolescentes(de 0 a 18 años de edad)
Para el tratamiento de hemorragias, la dosis en niños no difiere de la de los pacientes adultos. Para evitar hemorragias en niños menores de 6 años, se recomiendan dosis de 20 a 50 UI por kg de peso corporal de 3 a 4 veces por semana. La administración de ADVATE en niños (intravenosa) no difiere de la administración en adultos. Puede ser necesario un dispositivo de acceso venoso central (CVAD) para permitir infusiones frecuentes de productos de factor VIII.
Debido a la disminución del volumen de inyección de ADVATE reconstituido en 2 ml, el tiempo para reaccionar a las reacciones de hipersensibilidad durante una inyección se reduce aún más. Por tanto, se aconseja tener precaución durante la inyección de ADVATE reconstituido en 2 ml, especialmente en niños.
Cómo se administra ADVATE
ADVATE normalmente se inyecta en vena (vía intravenosa) por su médico o enfermera. Usted o cualquier otra persona pueden también administrar la inyección de ADVATE pero únicamente después de recibir la formación adecuada. Las instrucciones detalladas para su administración se describen al final de este prospecto.
Si usa más ADVATE del que debiera
Siga exactamente las instrucciones de administración de ADVATE indicadas por su médico. Consulte a su médico si tiene dudas.Si se inyecta una dosis mayor de ADVATE de la recomendada, consulte con su médico lo antes posible.
Si olvidó usar ADVATE
No se inyecte una dosis doble para compensar las dosis olvidadas. Adminístrese la siguiente inyección como está establecido y continúe como le había indicado su médico.
Si interrumpe el tratamiento con ADVATE
No deje de usar ADVATE sin consultarlo con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si se producen reacciones alérgicas(anafilácticas) repentinas ygraves, se debe parar inmediatamentela inyección. Contacte con su médico inmediatamente si tiene alguno de los siguientes síntomas iniciales de las reacciones alérgicas:
- erupción, sarpullido, ronchas y picor generalizado,
- hinchazón de los labios y la lengua,
- dificultad para respirar, jadeos, opresión en el pecho,
- sensación de malestar general,
- mareos y pérdida de consciencia.
Los síntomas graves, como dificultad para respirar y (casi) síncope, requieren un tratamiento temprano de emergencia.
En los niños que no han recibido tratamiento previo con medicamentos compuestos por factor VIII pueden producirse anticuerpos inhibidores (ver sección 2) muy frecuentemente (más de 1 de cada 10 pacientes); sin embargo, en los pacientes que han recibido tratamiento previo con factor VIII (más de 150 días de tratamiento), el riesgo es poco frencuente (menos de 1 de cada 100 pacientes). Si esto sucede, los medicamentos que toman usted o su hijo pueden dejar de funcionar correctamente y usted o su hijo pueden sufrir una hemorragia persistente. En ese caso, contacte con su médico inmediatamente.
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 pacientes)
Inhibidores del factor VIII (en los niños que no han recibido tratamiento previo con medicamentos compuestos por factor VIII).
Efectos adversos frecuentes(pueden afectar hasta a 1 de cada 10 pacientes)
Dolor de cabeza y fiebre
Efectos adversos poco frecuentes(pueden afectar hasta a 1 de cada 100 pacientes)
Inhibidores del factor VIII (en los pacientes que han recibido tratamiento previo con factor VIII [más de 150 días de tratamiento]), mareos, gripe, desfallecimiento, pulsaciones anormales, manchas rojas asociadas a picor, molestias en el pecho, cardenal en la zona de inyección, reacción en la zona de inyección, picor, aumento de la sudoración, sabor extraño en la boca, sofocos, migrañas, pérdidas de memoria, escalofríos, diarrea, náusea, vómitos, dificultad para respirar, dolor de garganta, infección de los vasos linfáticos, palidez, inflamación ocular, sarpullidos, sudoración excesiva, inflamación de pies y piernas, disminución del número de glóbulos rojos, aumento de un tipo de leucocitos (monocitos) y dolor en la parte superior del abdomen o parte inferior del pecho.
Relacionados con la cirugía
infección relacionada con el catéter, disminución del número de glóbulos rojos, hinchazón de las extremidades y las articulaciones, sangrado prolongado después de la retirada del drenaje, disminución de los niveles de factor VIII y hematoma postoperatorio
Relacionados con los dispositivos de acceso venoso central (CVAD)
Infección relacionada con catéter, infección sistémica y coágulo de sangre local en el lado del catéter.
Efectos adversos con frecuencia desconocida(no se puede estimar la frecuencia a partir de los datos disponibles)
Reacciones que pueden ser mortales (anafilaxis) y otras reacciones alérgicas (hipersensibilidad), trastornos generales (cansancio, falta de energía).
Efectos adversos adicionales en niños
Además del desarrollo de inhibidores en pacientes pediátricos sin tratamiento previo y las complicaciones asociadas al catéter, en los ensayos clínicos no se observaron diferencias específicas de la edad en los efectos adversos.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de ADVATE
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad se refiere al último día del mes que se indica.
Conservar en nevera (entre 2 °C y 8 °C).
No congelar.
Hasta la fecha de caducidad, el blister con el medicamento puede conservarse a temperatura ambiente (hasta 25 °C) por un periodo único no superior a 6 meses. En este caso, este medicamento caduca al final de este periodo de 6 meses o en la fecha de caducidad impresa en el blister, lo que ocurra antes. Por favor, anote en el envase del medicamento la fecha de finalización del periodo de conservación a temperatura ambiente de 6 meses. El medicamento no puede refrigerarse de nuevo después de conservarse a temperatura ambiente.
Conservar el blister con el medicamento en el embalaje exterior para protegerlo de la luz.
Este medicamento es para un solo uso. Elimine la solución no utilizada de forma apropiada.
Utilizar el medicamento inmediatamente tras la disolución completa del polvo.
No refrigerar el medicamento después de la preparación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de ADVATE
- El principio activo es octocog alfa (factor VIII humano de coagulación producido por tecnología de ADN recombinante). Cada vial de polvo contiene nominalmente 250, 500, 1000 o 1500 UI de octocog alfa.
- Los demás componentes son manitol, cloruro de sodio, histidina, trealosa, cloruro de calcio, trometamol, polisorbato 80 y glutatión (reducido)
Vial de disolvente: 2 ml de agua esterilizada para preparaciones inyectables
Aspecto del producto ycontenido del envase
ADVATE es un polvo desmenuzable de color blanco a blanquecino.
Después de su reconstitución, la solución es transparente, incolora y libre de partículas extrañas.
Titular de la autorización de comercialización
Takeda Manufacturing Austria AG
Industriestrasse 67
A-1221 Viena
Tel: +800 66838470
E-mail: [email protected]
Responsables de la fabricación
Baxalta Belgium Manufacturing SA
Boulevard René Branquart 80
B‑7860 Lessines
Bélgica
Fecha de la última revisión de este prospecto
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu/.
--------------------------------------------------------------------------------------------------------------------
Instrucciones para la preparación yadministración
ADVATE no se debe mezclar con otros medicamentos o disolventes.
Se recomienda encarecidamente registrar el nombre y el número de lote del producto cada vez que se administre ADVATE.
Instrucciones para la reconstitución
- No usar después de la fecha de caducidad que aparece en las etiquetas y en el envase.
- No lo utilice si la tapa del blister no está perfectamente sellada.
- No refrigerar la preparación después de la reconstitución.
- Si el medicamento se encuentra en la nevera, coger de la nevera el blister sellado (contiene viales de polvo y disolvente precargados con el sistema para su reconstitución) y dejarlo a temperatura ambiente (entre 15 °C y 25 °C).
- Lavar las manos con jabón y agua templada.
- Abrir el envoltorio de ADVATE quitando la tapa. Extraer el sistema BAXJECT III del blister.
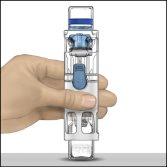
- Colocar ADVATE en una superficie plana con el vial de disolvente encima (Fig. 1). El vial de disolvente tiene una raya azul. No quitar el protector azul hasta que se le indique más adelante.
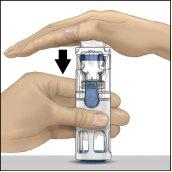
- Mientras sujeta ADVATE con una mano en el sistema BAXJECT III, presionar con fuerza el vial de disolvente con la otra mano hasta que el sistema quede totalmente contraído y el disolvente entre dentro del vial de ADVATE (Fig. 2). No inclinar el sistema hasta que haya finalizado la transferencia.
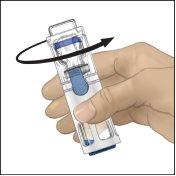
- Comprobar que ha finalizado la transferencia del disolvente. Agitar con suavidad hasta que todo el material se haya disuelto. Asegúrese de que el polvo ADVATE esté completamente disuelto, si no es así, toda la solución reconstituida no pasará a través del filtro del equipo. El medicamento se disuelve rápidamente (normalmente en menos de 1 minuto). Después de la reconstitución la solución debe ser transparente, incolora y no presenta partículas extrañas.
Fig.1 | Fig.2 | Fig.3 |
|
|
|
Instrucciones para la inyección
Se requiere una técnica aséptica durante la administración.
Para la administración se requiere el uso de una jeringa luer‑lock.
Nota importante:
- No intente administrar la inyección a menos que haya recibido una formación especial de su médico o enfermera.
- Inspeccione la solución preparada para detectar partículas o decoloración antes de la administración (la solución debe ser transparente, incolora y libre de partículas extrañas).
No utilice ADVATE si la solución no está totalmente transparente o no está disuelta completamente.
- Quitar el protector azul del sistema BAXJECT III. No introducir aire en la jeringa. Conectar la jeringa al sistema BAXJECT III.
- Invertir el sistema (el vial con la solución reconstituida en la parte superior). Introducir la solución reconstituida en la jeringa, tirando del émbolo hacia atrás lentamente.
- Desconectar la jeringa.
- Conectar una aguja de perfusión con aletas a la jeringa. Inyectar intravenosamente. La solución se debe administrar lentamente, a una velocidad determinada de acuerdo al nivel de comodidad del paciente, que no exceda de 10 ml por minuto. Debe tomarse el pulso antes y durante la administración de ADVATE. Si observa un aumento significativo del pulso, debe reducir la velocidad de administración o interrumpir temporalmente la inyección, normalmente esto permite que los síntomas desaparezcan en seguida (ver sección 4 “Posibles efectos adversos”).
- Elimine la solución no utilizada de forma apropiada.
--------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
Tratamiento bajo demanda
En el caso de los episodios hemorrágicos posteriores, la actividad de factor VIII no debe ser inferior al nivel de actividad plasmática dada (en % del normal o UI/dl) en el periodo correspondiente. Se puede utilizar la siguiente tabla como guía de dosificación en episodios hemorrágicos y cirugía.
La dosis y frecuencia de la administración se debe adaptar a la respuesta clínica en cada caso individual. En determinadas circunstancias (ej.: presencia de un título bajo de inhibidor) pueden ser necesarias dosis mayores a las calculadas mediante la fórmula.
Grado de hemorragia / tipo de procedimiento quirúrgico | Nivel de Factor VIII requerido (% oUI/dl) | Frecuencia de dosis (horas) / duración de la terapia (días) |
Hemorragia Hemartrosis incipiente o hemorragia muscular u oral. Hemartrosis más extensa, hemorragia muscular o hematoma. Hemorragia con riesgo vital. | 20 – 40 30 – 60 60 – 100 | Repetir la inyección cada 12 a 24 horas (cada 8 a 24 horas en los pacientes menores de 6 años) al menos 1 día hasta que el episodio hemorrágico, según indique el dolor, se resuelva o se logre la curación. Repetir la inyección cada 12‑24 horas (cada 8 a 24 horas en los pacientes menores de 6 años) durante 3 a 4 días o más, hasta que cese el dolor y la incapacidad aguda. Repetir la inyección cada 8 a 24 horas (cada 6 a 12 horas en los pacientes menores de 6 años) hasta superar el peligro. |
Cirugía MenorIncluyendo extracción dental. Mayor | 30 – 60 80 – 100 (pre y postoperatorio) | Cada 24 horas (cada 12 a 24 horas en los pacientes menores de 6 años), al menos 1 día, hasta lograr la curación. Repetir la inyección cada 8‑24 horas (cada 6 a 24 horas en los pacientes menores de 6 años) hasta que se consiga una curación adecuada de la herida, y luego al menos otros 7 días de terapia para mantener una actividad de factor VIII del 30% al 60% (UI/dl). |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ADVATE 500 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 1.000 UIPrincipio activo: factor de coagulación VIIIFabricante: Takeda Manufacturing Austria AgRequiere recetaForma farmacéutica: INYECTABLE, 1.500 UIPrincipio activo: factor de coagulación VIIIFabricante: Takeda Manufacturing Austria AgRequiere recetaForma farmacéutica: INYECTABLE, 1000 UI - tras reconstitución en 2 ml de agua para inyectables la dosis es de 500 UI/mlPrincipio activo: factor de coagulación VIIIFabricante: Takeda Manufacturing Austria AgRequiere receta
Médicos online para ADVATE 500 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ADVATE 500 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes