
ADRENALINA AGUETTANT 0,1 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar ADRENALINA AGUETTANT 0,1 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Adrenalina Aguettant 0,1 mg/ml, solución inyectable en jeringa precargada
(De ahora en adelante “Adrenalina inyectable”)
Epinefrina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Adrenalina inyectable y para qué se utiliza
- Qué necesita saber antes de empezar a usar Adrenalina inyectable
- Cómo usar Adrenalina inyectable
- Posibles efectos adversos
- Conservación de Adrenalina inyectable
- Contenido del envase e información adicionall
1. Qué es Adrenalina inyectable y para qué se utiliza
Adrenalina inyectable pertenece a un grupo de medicamentos denominados agentes adrenérgicos y dopaminérgicos.
Este medicamento se utiliza para:
- Tratamiento del paro cardíaco (pérdida inesperada de la función cardíaca, la respiración y el conocimiento).
- Tratamiento de anafilaxia aguda en adultos (shock o colapso graves producidos por una reacción alérgica grave).
2. Qué necesita saber antes de que le administren Adrenalina inyectable
No le deben administrarAdrenalina inyectable
- si es alérgico (hipersensible) a alguno de los componentes de este medicamento (incluidos en la sección 6), cuando se disponga de una presentación alternativa de adrenalina o un vasopresor alternativo.
Advertencias y precauciones
Adrenalina inyectable está indicada para el tratamiento de urgencia. Es necesaria supervisión médica continua después de la administración.
Precauciones de uso
El riesgo de efectos adversos aumenta si usted:
- presenta antecedentes médicos de hipertiroidismo (enfermedad de la glándula tiroidea);
- presenta insuficiencia renal grave;
- sufre de hipercalcemia (aumento de la concentración de calcio en la sangre);
- sufre de hipopotasemia (disminución de la concentración de potasio en la sangre);
- presenta diabetes mellitus;
- presenta una cardiopatía o hipertensión arterial;
- presenta daño cerebral o endurecimiento de las arterias del cerebro;
- presenta glaucoma (aumento de la presión en el ojo);
- presenta trastornos de la próstata;
- es un paciente de edad avanzada;
- está embarazada.
Uso en deportistas
Se informa a los deportistas que este medicamento contiene adrenalina, que puede producir un resultado positivo en las pruebas de control de dopaje
Uso de Adrenalina inyectable con otros medicamentos
Informe a su médico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Los medicamentos que pueden interaccionar con Adrenalina inyectable incluyen:
- anestésicos halogenados volátiles (gas utilizado durante la anestesia);
- ciertos antidepresivos;
- fármacos para tratar la hipertensión arterial, las afecciones del corazón;
- fármacos para tratar la diabetes.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de que le administren este medicamento.
Conducción y uso de máquinas
Recibir Adrenalina inyectable no afecta a su capacidad para conducir o usar máquinas.
Adrenalina inyectable contiene sodio
Este medicamento contiene 35,4 mg de sodio (componente principal de la sal de mesa/para cocinar) en cada jeringa. Esto equivale al 1,77% de la ingesta diaria máxima de sodio recomendada para un adulto.
3. Cómo se administra Adrenalina inyectable
Adrenalina inyectable le será administrada por su médico, enfermero o auxiliar sanitario. Ellos tendrán que decidir la cantidad correcta para usted, así como cuándo y cómo debe administrarse.
En caso de reacciones alérgicas potencialmente mortales (anafilaxia aguda):
A los adultosse les administrará una dosis de 0,05 mg (0,5 ml de la solución 1:10.000 de adrenalina) tantas veces como sea necesario hasta obtener la respuesta deseada.
En caso de paro cardíaco:
Adultos:se administra 1 mg (10 ml de la solución 1:10.000 de adrenalina) en una vena o en el hueso cada 3-5 minutos hasta que el corazón comience a funcionar.
Niños con más de 5 kg:se administran 10 microgramos/kg (0,1 ml/kg de la solución 1:10.000 de adrenalina) en una vena o en un hueso cada 3-5 minutos hasta que el corazón comience a funcionar.
Este medicamento no es adecuado para administrar una dosis menor de 0,5 ml y por tanto no debería ser usado en recién nacidos o niños con un peso corporal menor de 5 kg.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Se han notificado los siguientes efectos adversos:
- ansiedad;
- disnea (dificultad para respirar);
- nerviosismo;
- miedo;
- sudoración;
- palpitaciones (latido cardíaco irregular o acelerado);
- taquicardia (frecuencia cardíaca aumentada);
- palidez;
- temblores;
- debilidad;
- mareos;
- dolor de cabeza;
- náuseas;
- vómitos;
- frío en las extremidades;
- alucinaciones;
- síncopes;
- hiperglucemia (niveles altos de azúcar en la sangre);
- hipopotasemia (niveles bajos de potasio en la sangre);
- acidosis metabólica (aumento de la acidez en la sangre);
- midriasis (dilatación de la pupila).
A dosis elevadas o en pacientes sensibles a la adrenalina, los efectos adversos son:
- arritmia cardíaca (latidos cardíacos irregulares/paro cardíaco);
- hipertensión arterial (con riesgo de hemorragia cerebral);
- vasoconstricción (estrechamiento de los vasos sanguíneos, por ejemplo, cutáneos, en las extremidades o en los riñones);
- angina de pecho aguda;
- riesgo de infarto agudo de miocardio.
Las inyecciones locales repetidas pueden producir necrosis (daño tisular) en los lugares de inyección, como resultado de la constricción vascular (constricción de los vasos sanguíneos).
En todos los casos, es necesaria la supervisión médica tras la administración de Adrenalina inyectable.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento
5. Conservación de Adrenalina inyectable
Mantener este medicamento fuera de la vista y del alcance de los niños.
No debe recibir este medicamento después de la fecha de caducidad que aparece en la etiqueta. La fecha de caducidad es el último día del mes que se indica. Su médico o enfermero verificarán esto.
Conservar en la bolsa de aluminio para protegerla de la luz y el oxígeno.
No conservar a temperatura superior a 25°C.
No abrir la bolsa de aluminio hasta que vaya a usarse.
Después de abrir la bolsa, el producto se debe usar inmediatamente.
No congelar.
No utilice objetos afilados para abrir la bolsa.
No debe recibir Adrenalina inyectable si ha sido parcialmente utilizada o muestra indicios visibles de deterioro.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Adrenalina inyectable
El principio activo es epinefrina (adrenalina):
Cada ml de solución inyectable contiene 0,1 mg de epinefrina (adrenalina), en forma de tartrato de adrenalina.
Cada jeringa precargada de 10 ml contiene 1 mg de epinefrina (adrenalina), en forma de tartrato de adrenalina.
Los demás componentes son cloruro de sodio, ácido clorhídrico, hidróxido sódico, agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Adrenalina inyectable es una solución incolora transparente en una jeringa precargada de polipropileno de 10 ml, envasada individualmente en un blíster transparente y envuelta en una bolsa de aluminio.
Las jeringas precargadas están disponibles en cajas de 1 y 10 jeringas.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización:
Laboratoire Aguettant
1, rue Alexander Fleming
69007 LYON
FRANCIA
Responsable de la fabricación:
Laboratoire Aguettant
1, rue Alexander Fleming
69007 LYON
FRANCIA
Laboratoire Aguettant
Lieu Dit Chantecaille
07340 Champagne
FRANCIA
Representante Local:
AGUETTANT IBERICA
C/ Baldini Reixac, 4-8, Torre I, 4º08028, Barcelona-España
Fecha de la última revisión de este prospecto: Julio 2024
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
Esta información está destinada únicamente a profesionales del sector sanitario:
La adrenalina intravenosa sólo debe ser administrada por aquellas personas que tengan experiencia en el uso y ajuste de la dosis de vasopresores en su práctica clínica habitual.
Reanimación cardiopulmonar:
10 ml de solución 1:10.000 (1 mg), por vía intravenosa o intraósea, repetida cada 3-5 minutos hasta que vuelva la circulación espontánea.
El uso endotraqueal debe usarse únicamente como último recurso si ninguna otra vía de administración es accesible, a una dosis de 20 a 25 ml de solución 1:10.000 (2 a 2,5 mg).
En paro cardiaco tras una intervención quirúrgica cardiaca, la adrenalina debe administrarse por via intravenosa en dosis de 0,5ml o 1 ml de solución 1:10.000 (50 o 100 microgramos) con mucha precaución y ajustando la dosis hasta lograr el efecto.
Anafilaxia aguda:
Ajustar la dosis usando bolos intravenosos de 0,5ml de solución 1:10.000 (0,05 mg) según la respuesta.
Adrenalina 0,1 mg/ml (1:10.000) solución inyectable en jeringa precargada no se recomienda para su uso intramuscular en casos de anafilaxia aguda. Para la administración intramuscular debe utilizarse una solución de 1 mg/ml (1:1000).
Población pediátrica:
Este medicamento no es adecuado para administrar una dosis menor de 0,5 ml y por lo tanto no debe ser usado por via intravenosa o intraósea, en recién nacidos y niños con un peso corporal menor de 5 kg.
Paro cardiaco en niños:
Vía intravenosa o intraósea (sólo para niños de más de 5kg): 0,1 ml/kg de solución 1:10.000 (10 microgramos/kg) hasta una dosis única máxima de 10 ml de solución 1:10.000 (1 mg), repetida cada 3-5 minutos hasta que vuelva la circulación espontánea.
El uso endotraqueal (en cualquier rango de peso) debe usarse, únicamente como último recurso si ninguna otra vía de administración es accesible, a una dosis de 1 ml/kg de solución 1:10 000 (100 microgramos/kg) hasta una dosis única máxima de 25 ml de solución 1:10.000 (2,5 mg).
Siga estrictamente el siguiente protocolo:
La jeringa precargada está destinada a un único paciente. Deseche la jeringa después de su uso. No reutilizar.
El producto debe inspeccionarse visualmente para detectar partículas y cambios de color antes de su administración. Solo debe usarse la solución transparente, incolora y sin partículas ni precipitados.
El producto no debe usarse si la bolsa o el blíster han sido abiertos o si el precinto de seguridad de la jeringa (capa de plástico en la base del capuchón de la punta) está roto.
- Abra la bolsa de aluminio con la mano usando solamente la(s) hendidura(s).
No utilice objetos afilados para abrir la bolsa.
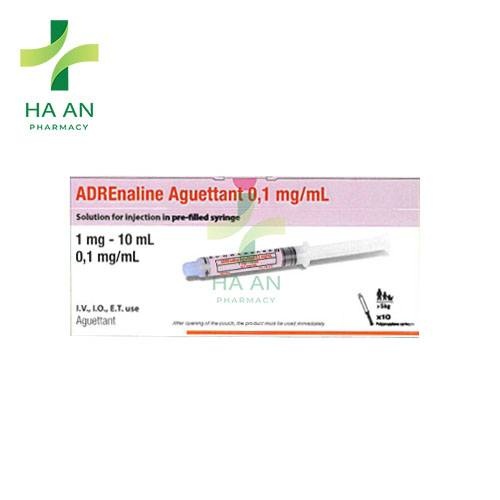
- Saque la jeringa precargada del blíster estéril.
- Pulse el émbolo para liberar el tapón. El proceso de esterilización puede haber provocado que el tapón se adhiera al cuerpo de la jeringa.
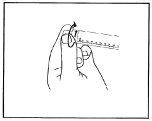
- Desenrosque el capuchón de la punta para romper el precinto.No toque la conexión de Luer expuesta para evitar la contaminación.
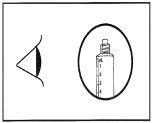
- Compruebe que el precinto de la jeringa se ha quitado por completo. En caso contrario, vuelva a colocar el capuchón y gírelo nuevamente.
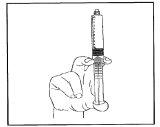 Saque el aire empujando el émbolo con suavidad.
Saque el aire empujando el émbolo con suavidad.
- Conecte la jeringa al dispositivo de acceso vascular o a la aguja.
Empuje el émbolo para inyectar el volumen necesario.
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ADRENALINA AGUETTANT 0,1 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, Adrenalina base 1 mg/mlPrincipio activo: AdrenalinaFabricante: B Braun Medical S.A.Requiere recetaForma farmacéutica: INYECTABLE, 1 mg/mlPrincipio activo: AdrenalinaFabricante: Laboratorios Basi Industria Farmaceutica S.A.Requiere recetaForma farmacéutica: INYECTABLE, 1 mg/mlPrincipio activo: AdrenalinaFabricante: Bradex S.A.Requiere receta
Médicos online para ADRENALINA AGUETTANT 0,1 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ADRENALINA AGUETTANT 0,1 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











